
A decade after the first-in-human transcatheter mitral valve edge-to-edge repair, the first transcatheter mitral valve replacement (TMVR) was performed in 2012 by Lars Søndergaard (Copenhagen, Denmark) using the CardiAQ™ valve system (Edwards Lifesciences [previously CardiAQ Valve Technologies], Irvine, CA, USA) in patients with severe mitral regurgitation (MR)1. Seven years later, with fewer than 1,000 transcatheter mitral valve replacements (TMVR) performed worldwide, we are in the midst of performing randomised controlled trials comparing mitral valve surgery and TMVR in the USA, and potentially obtaining CE mark approval for TMVR systems in the near future. Prediction of procedural outcomes is a pivotal milestone towards integration of TMVR into clinical practice, as it may lead to a better selection of patients and influence the design of future clinical trials and iterative devices.
However, there has been a dearth of clinical data regarding prediction of clinical outcomes following TMVR. In the current issue of EuroIntervention, Badhwar et al aimed to define predictors of adverse outcomes following TMVR based on the one-year outcomes of the CE mark global feasibility clinical study of the Tendyne transcatheter mitral valve system (Abbott Vascular, Santa Clara, CA, USA)2.
The trial was initiated five years ago as a single-arm open-label trial, that included patients with moderate-severe and severe MR deemed at prohibitive risk for surgery from 24 participating centres (3 in Australia, 8 in Europe and 13 in the USA). In a recent report of the first 100 patients enrolled in the Tendyne Global Feasibility trial, there were no intraprocedural deaths, one case of major apical bleeding, and no cases of conversion to surgery or need for cardiopulmonary bypass3. The 30-day mortality and stroke rates were 6% and 2%, respectively. At one year, freedom from all-cause mortality was 72.4%, with 84.6% of deaths related to cardiac causes. Among survivors, 88.5% were in New York Heart Association (NYHA) functional Class I/II, with a significant improvement in six-minute walk distance (p<0.0001) and quality of life (p=0.011).
In the current study, Badhwar et al use a composite endpoint of one-year mortality and heart failure hospitalisation (HFH) to identify independent predictors of outcomes following TMVR with the Tendyne device2. Using univariate and multivariate logistic regression analyses, they found that prior percutaneous coronary intervention (PCI) (OR 6.44, p=0.028), renal insufficiency (OR 8.15, p=0.021) and hypertension (OR 20.7, p=0.069) independently predicted the composite of mortality and HFH at one year. In addition, preprocedural severe MR, as compared to moderate-severe MR, predicted freedom from mortality and HFH (OR 0.16, p=0.032). The findings presented by Badhwar et al are novel and important, as they shed new light on risk stratification of MR patients considered for TMVR.
The population of patients represented in the study of Badhwar et al is at extremely high risk for intervention. Absence of procedure-related mortality in these patients is thus encouraging and should open the door to larger TMVR clinical trials. Given the well-recognised poor prognosis of severe MR patients with a conservative treatment4, a key issue to be addressed in future trials will be to delineate the profiles of candidates who will benefit from transcatheter valve repair, as compared with transcatheter valve replacement. As regards the prognostic predictors found in the current study, renal failure was previously shown to predict mortality also following transcatheter mitral valve repair5. In contrast, the prognostic role of coronary artery disease in patients undergoing transcatheter mitral valve repair remains equivocal6. Eventually, the above predictors detected by Badhwar et al might potentially contribute to characterisation of the patients suitable for each transcatheter strategy.
One of the intriguing findings in the current study is that severe MR (assessed by the core laboratory as effective regurgitant orifice area [EROA] ≥0.3 cm2 or regurgitant volume ≥45 ml, n=16), as compared to less-than-severe MR (n=35), was identified as an independent predictor of decreased mortality, despite a more pronounced left ventricular (LV) remodelling. This finding might be explained by the concept recently suggested by Grayburn et al, distinguishing between proportionate versus disproportionate secondary MR7. Accordingly, a proportionate increase in EROA and left ventricular end-diastolic dilatation does not necessarily translate into a worse prognosis, but rather delineates a group of patients who are more likely to benefit from a strategy that includes left ventricular end-diastolic volume (LVEDV) reduction. The authors suggested that the Tendyne stabilisation system (i.e., a polyethylene tether affixed with an epicardial pad) might induce LV reverse remodelling along with the MR reduction resulting from the prosthesis.
Along with the interest of the findings described by Badhwar et al, several major limitations should be pointed out upon interpretation of the study. First, the Tendyme feasibility trial was a single-arm, non-controlled trial and as such did not provide any comparison with alternative treatment. Secondly, while the whole cohort univariate logistic regression model remains of descriptive value, the multivariate analysis included only half of the cohort (n=51) due to missing data. Multicollinearity assessment markedly reduced the number of variables included in the multivariate model from 21 to 9; however, the event-to-variable ratio in such a small sample size still raises a concern of sparse data bias. Thus, the suggested predictors in the current study should be interpreted with caution and will have to be supported by more robust evidence. Among many other ongoing TMVR trials, the randomised clinical trial to evaluate the safety and effectiveness of using the Tendyne Mitral Valve System for the treatment of symptomatic mitral regurgitation (SUMMIT), in patients with secondary functional MR, will hopefully clarify unanswered questions regarding TMVR outcomes (Figure 1).
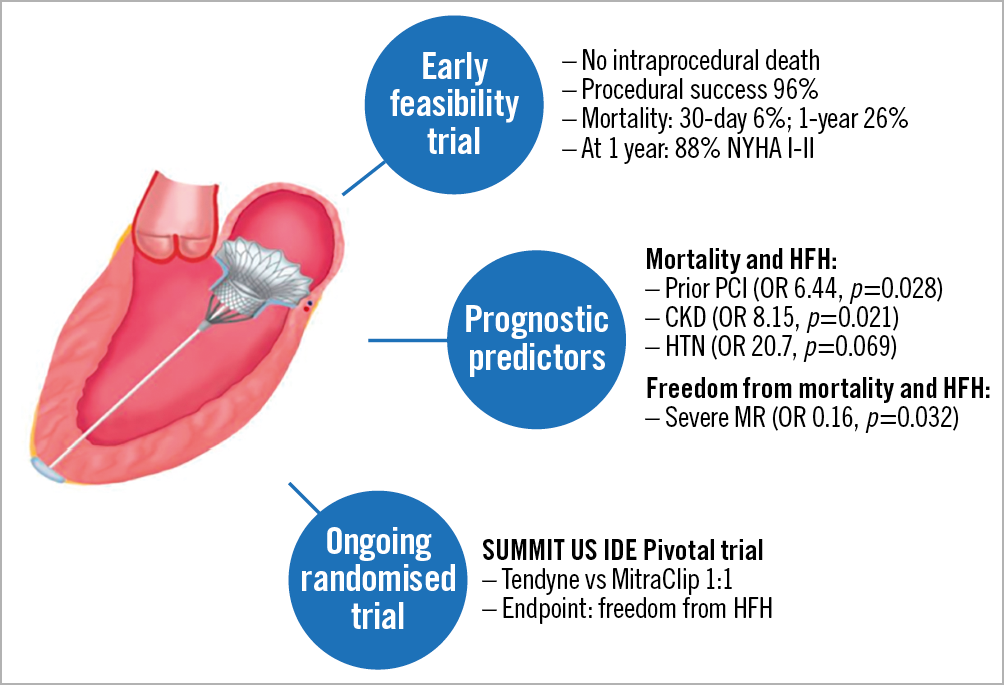
Figure 1. Current data on the Tendyne transcatheter mitral valve system. CKD: chronic kidney disease; HFH: heart failure hospitalisation; HTN: hypertension; MR: mitral regurgitation; PCI: percutaneous coronary intervention; SUMMIT: Tendyne Mitral Valve System for the treatment of symptomatic mitral regurgitation
Finally, the current report of Badhwar et al2 provides us with a notion regarding potential predictors of outcomes following transapical TMVR. While the presented data are limited by the obvious caveats of an early feasibility study, the present findings should prompt further clinical study to assess the efficacy of TMVR in high-risk severe MR patients, including those with advanced LV remodelling.
It is difficult to make predictions, particularly about the future.
Niels Bohr.
Conflict of interest statement
The authors have no conflicts of interest to declare.

