Abstract
Background: In the MITRA-FR trial, transcatheter mitral valve repair (TMVR) was not associated with a 2-year clinical benefit in patients with secondary mitral regurgitation (SMR).
Aims: This landmark analysis aimed at investigating a potential reduction of the hospitalisation rate for heart failure (HF) between 12 and 24 months after inclusion in the MITRA-FR trial in patients randomised to the intervention group (TMVR with the MitraClip device), as compared with patients randomised to the control group (guideline-directed medical therapy [GDMT]).
Methods: The MITRA-FR trial randomised 307 patients with SMR for TMVR on top of GDMT (TMVR group; n=152) or for GDMT alone (control group; n=155). We conducted a 12-month landmark analysis in surviving patients who were not hospitalised for HF within the first 12 months of follow-up. The primary endpoint was the 1-year cumulative number of HF hospitalisations.
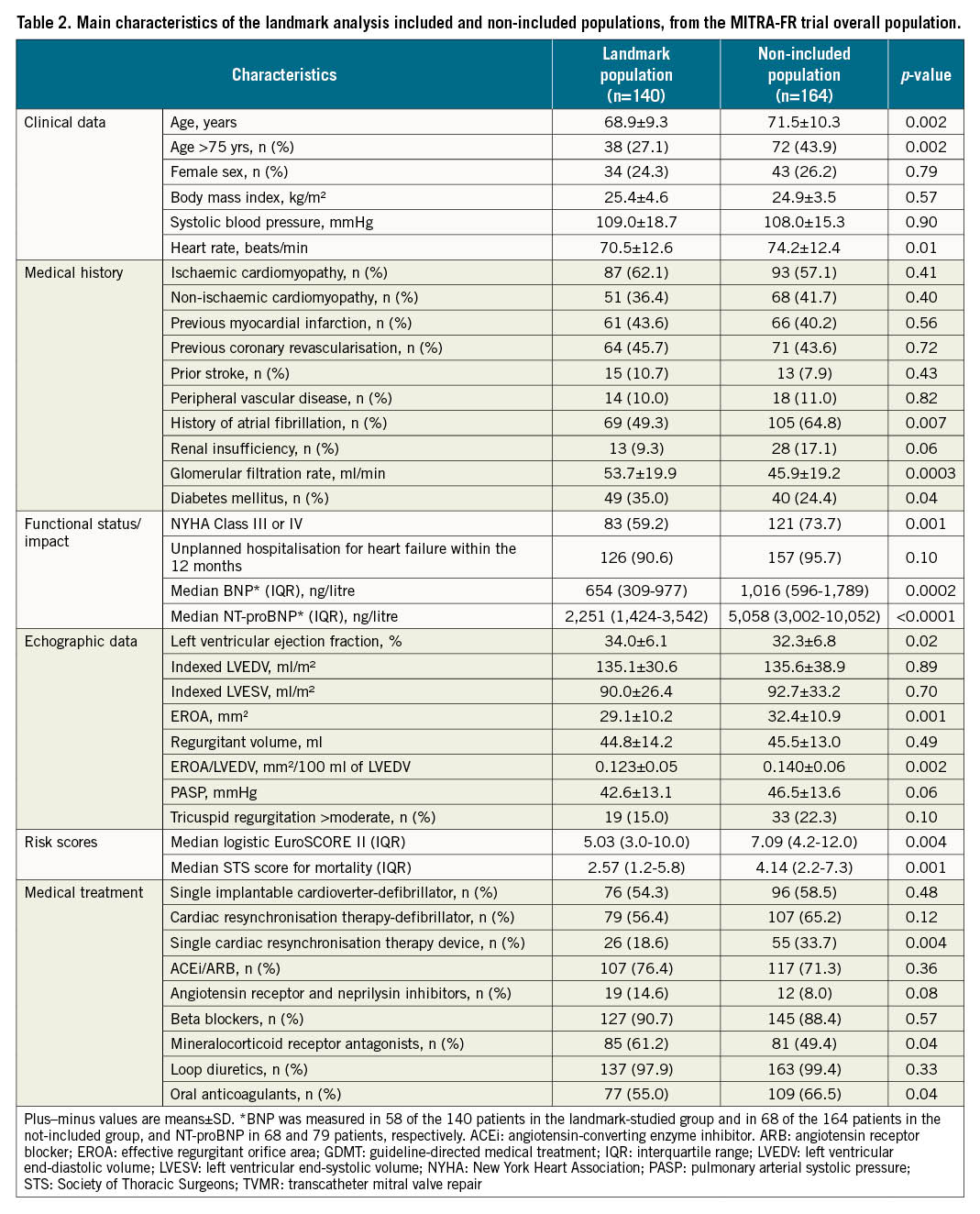
Results: A total of 140 patients (TMVR group: 67; GDMT group: 73) were selected for this landmark analysis with similar characteristics at inclusion in the trial. The primary endpoint was 28 events per 100 patient-years in the TMVR group, as compared with 60 events per 100 patient-years in the GDMT group (hazard ratio [HR] 0.46, 95% confidence interval [CI]: 0.20-1.02; p=0.057).
Conclusions: In this landmark analysis of the MITRA-FR trial, the cumulative rate of HF hospitalisation between 12 and 24 months among patients treated with TMVR on top of GDMT was approximately half as many as those of patients treated with GDMT alone, a difference which did not reach statistical significance in the setting of a low number of events.
Introduction
The benefit of transcatheter mitral valve repair (TMVR) in the management of severe and symptomatic secondary mitral regurgitation (SMR) remains a subject of debate since the simultaneous publications of two large randomised trials12. On one hand, percutaneous repair with the MitraClip (Abbott Vascular) device for the severe Functional/secondary mitral Regurgitation (MITRA-FR) trial found no difference in the 24-month composite of all-cause death and unplanned hospitalisation rate for heart failure (HF)3. On the other hand, the Cardiovascular Outcomes Assessment of the MitraClip Percutaneous Therapy for heart failure patients with functional mitral regurgitation (COAPT) trial highlighted a clear benefit of TMVR with the MitraClip device added to optimal guideline-directed medical therapy (GDMT), allowing a significant reduction of the annualised rate of all heart failure hospitalisations within 2 years of inclusion2. Many assumptions were made to explain these discrepancies, such as the presence of a central eligibility committee in COAPT and not in MITRA-FR, differences in the medical treatment management before randomisation and during the follow-up, and, mostly, differences in the cardiovascular profile of patients included in these two trials45. The 2-year results of the MITRA-FR study showed a mismatch between a constant and similar mortality rate in the intervention and the control groups after 1 year of follow-up, contrasting with a slight reduction of the HF hospitalisation rate in the intervention group (though not reaching statistical significance)3. Since a 12-month delay is long enough for identification of the patients with the most severe symptoms and is a commonly accepted threshold for the assessment of the potential benefit of any procedure, we herein aimed to analyse a potential delayed benefit of the MitraClip device in the MITRA-FR trial patients who did not experience any component of the primary objective, i.e., death from any cause or unplanned hospitalisation for HF, using a 12-month landmark analysis.
Methods
The MITRA-FR trial
The methods of the MITRA-FR trial have been previously thoroughly presented16. Briefly, 304 patients with severe (regurgitant volume >30 mL/beat or an effective regurgitant orifice area [EROA] >20 mm²) and symptomatic (New York Heart Association [NYHA] Functional Class ≥II and at least one hospitalisation for HF decompensation in the preceding 12 months) SMR despite GDMT were prospectively randomised at 37 French centres in a 1:1 ratio in 2 groups: pursuit of the GDMT alone (“GDMT group”) or TMVR in addition to the GDMT (“TMVR group”).
The screening transthoracic and transoesophageal echocardiograms performed prior to randomisation were analysed by an independent centralised core laboratory. All serious adverse events were adjudicated by an independent events validation committee blinded to treatment assignment. The study was conducted and coordinated by the Clinical Investigation Centre of Lyon, which is an academic research organisation based at Hospices Civils de Lyon (INSERM 1407) and mainly financed by an academic grant from the French Ministry of Health. The MITRA-FR trial was approved by a central ethics committee and the French National Agency for Medicines and Health Products Safety and was conducted in accordance with the provisions of the Declaration of Helsinki.
The primary efficacy endpoint of the MITRA-FR trial was the composite of all-cause death and unplanned HF hospitalisation. Results at 12 and 24 months were previously reported13.
Study device and procedure
The evaluated technique was TMVR with the MitraClip device, as previously described7. Technical proctoring from Abbott Vascular was provided for all procedures.
Rationale of this analysis
One possible explanation of the divergent results between the MITRA-FR and COAPT trials is that the MITRA-FR patients were at a later stage of HF, with less severe SMR and left ventricular (LV) remodelling and dysfunction too advanced to benefit from any therapy. Thus, we assumed that we should exclude from the present analysis the patients who died or were hospitalised for HF during the first year. Therefore, we analysed the outcomes between 12 and 24 months after randomisation for the selected subgroup of patients who did not reach the MITRA-FR primary endpoint at 12 months. The main characteristics of the two groups (TMVR and GDMT) of this landmark analysis were compared. We also compared characteristics of the patients selected in this landmark analysis with those who either died or were hospitalised for HF within the first year of inclusion in the trial.
In addition, considering recent publications about the pathophysiology of SMR, we analysed patients according to the ratio of preprocedural EROA to the left ventricular end-diastolic volume (LVEDV)89. Major adverse cardiac events were defined as the composite of death, stroke, myocardial infarction, or unplanned HF hospitalisation.
To allow cross comparison with the COAPT trial, the primary outcome of this landmark analysis was the annualised cumulative rate of HF hospitalisations between 12 and 24 months after inclusion in the MITRA-FR trial. Secondary outcomes were the composite of all-cause death and unplanned HF hospitalisation between 12 and 24 months after inclusion in the MITRA-FR trial, and the individual components of this composite outcome.
As a sensitivity analysis, the analysis was repeated after adjustment for prognostically important patient characteristics (age, baseline NYHA Class, type of cardiomyopathy, LV ejection fraction). In order to address the competing risk of death, we performed a “cause-specific hazard” model and a Fine-Gray model (subdistribution hazard model).
Last, we used the same statistical analysis on a landmark analysis selecting all 12-month surviving MITRA-FR patients, whether they were hospitalised or not during the first year of follow-up.
Statistical analysis
Between-group comparisons used the Fisher's exact test for qualitative variables and the Wilcoxon rank-sum test for quantitative variables. Efficacy endpoints were analysed with the Cox’s proportional-hazards regression model stratified on centre to estimate the treatment effect (hazard ratio [HR] and confidence interval [CI]) on time-to-event data10. Kaplan-Meier survival curves were drawn.
The distribution of the number of hospitalisations for HF per patient was compared between the two treatment groups with a Wilcoxon rank-sum test. The rate of HF hospitalisations per patient-year was calculated and compared between the two treatment groups with a negative binomial generalised linear regression model accounting for overdispersed data and correlated events, using the log of follow-up time as an offset. In addition, the competitive risk of death with the recurrent events process was explored using a joint frailty model with a Weibull distribution for modelling the baseline hazard11. Changes from baseline were compared between the two groups with the paired Student’s t-test, or the Wilcoxon rank-sum test in case of non-normality of the distributions. A two-sided p-value of less than 0.05 indicates statistical significance. All statistical analyses were performed using SAS software version 9.4 (SAS Institute) in a Windows environment. All statistical analyses were performed by the statistical department of the Hospices Civils de Lyon.
Results
Results of the MITRA-FR trial
The results of the MITRA-FR were previously published: 307 patients with HF and severe (mean EROA: 31±11 mm²; regurgitant volume 45±13 ml) SMR were randomised to the intervention (152 patients) or to the control (155 patients) group, with similar characteristics and guideline-directed medical treatment at baseline (except for history of myocardial infarction, which was more common in the intervention group). Implantation was not attempted in eight patients randomised to the intervention group. Results showed no differences at the primary endpoint (a composite of death from any cause or unplanned hospitalisation for heart failure) at 12 and 24 months13.
Population selected for the landmark analysis
From the MITRA-FR trial overall population, 161 patients reached the MITRA-FR primary endpoint (death: 71 patients; unplanned hospitalisation for HF: 146 patients) and three patients withdrew their consent within the first 12 months. Thus, 140 patients (TMVR group: 67 patients; GDMT group: 73 patients) formed this 12-month landmark analysis population (Table 1). Clinical, biological and echocardiographic data at inclusion in the MITRA-FR trial were similar in the 2 groups, except for history of myocardial infarction. The main echographic data at 12 months in the TMVR and GDMT groups are presented in Supplementary Table 1.
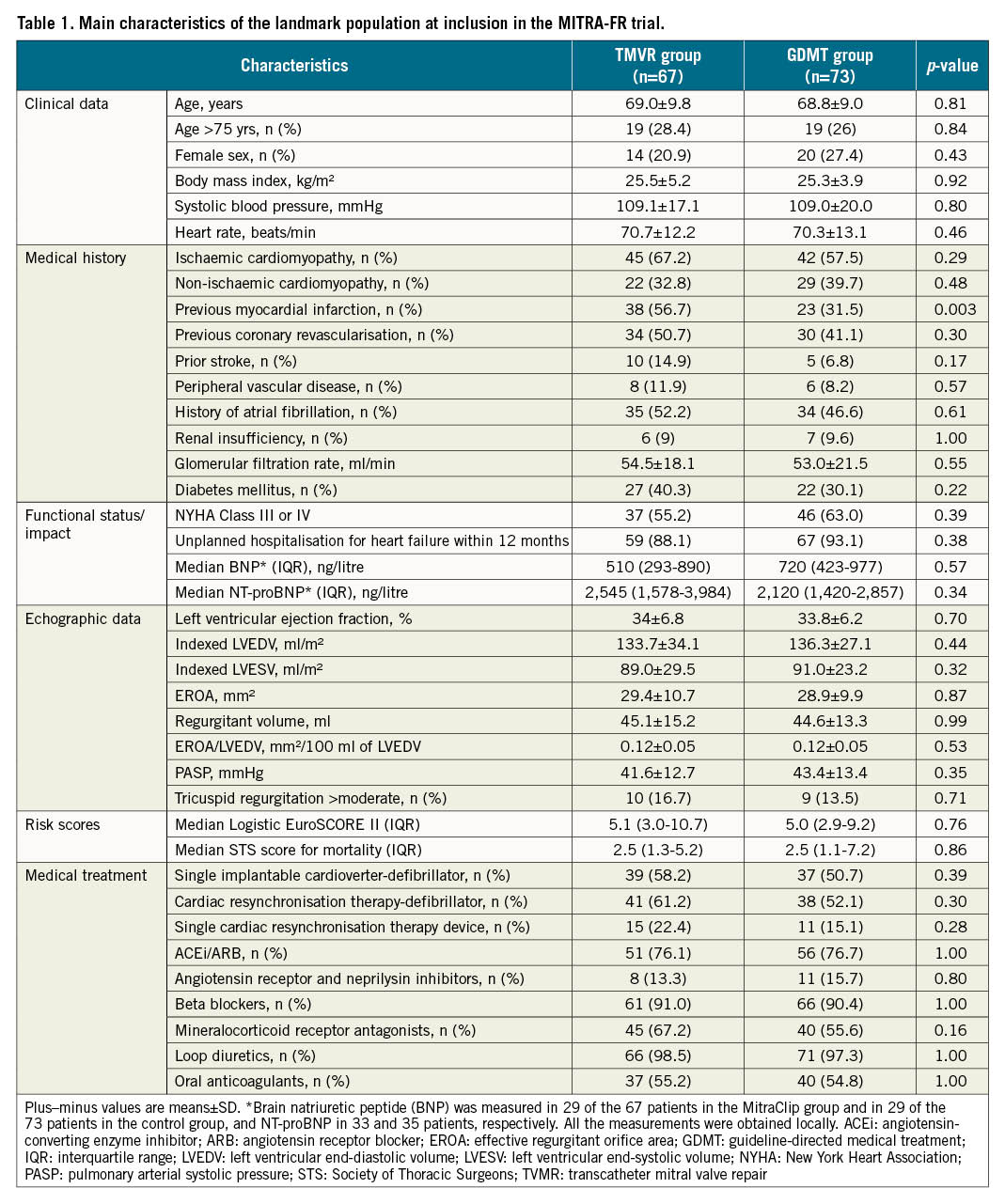
The patients of this landmark analysis presented with less severe characteristics than the patients who died or were hospitalised for HF during the first year (Table 2). Selected patients were younger (age 68±9 vs 71±10; p=0.002), less symptomatic (NYHA Class I or II: 41% vs 26%; p=0.0014), with lower median (1st-3rd quartile) risk scores (logistic EuroSCORE II: 5.0 [3.0-10.0] vs 7.0 [4.2-12.0]; p=0.004; STS mortality score: 2.6 [1.2-5.8] vs 4.1 [2.2-7.3]; p=0.001), less renal impairment (glomerular filtration rate 53±19 vs 46±19 ml/min; p=0.0003) and less elevated brain natriuretic peptide (BNP) levels with a median (1st-3rd quartile) of 654 ng/l (309-977) vs 1,016 ng/l (596-1,789); p=0.0002. Furthermore, the echocardiographic data also highlighted the less severe profile of included patients who harboured a better LV function (LV ejection fraction 34±6% vs 32±7%; p=0.02), less severe mitral regurgitation (EROA 29±10 vs 32±11 mm²; p=0.0019), and a lower EROA/LVEDV ratio (0.12±0.05 vs 0.14±0.06 mm²/100 ml of LVEDV; p=0.002) than non-included patients, with similar end-diastolic volumes (indexed LVEDV 135±31 vs 135±39 ml/m²; p=0.89).
Outcome
The predicted cumulative hospitalisation rate for HF between 12 and 24 months after inclusion in the MITRA-FR trial was 27.6 events per 100 patient-years in the TMVR group as compared with 60.0 events per 100 patient-years in the GDMT group (HR 0.45, 95% CI: 0.20-1.02; p=0.057) (Figure 1). The number of HF hospitalisations was 0 in 83.5% of the TMVR patients (vs 69.8% of the GDMT patients), 1 in 8.9% (vs 15.0%), 2 in 5.9% (vs 13.7%) and 3 in 1.4% (vs 1.3%). Because of a limited number of events, the joint frailty model that took into account the competitive risk of death for the analysis of the cumulative rate of HF hospitalisation did not converge. Between 12 and 24 months after inclusion in the MITRA-FR trial, the composite secondary outcome of death from any cause or unplanned HF hospitalisation occurred in 14 patients (20.1%) in the TMVR group and in 24 patients (32.9%) in the GDMT group (HR 0.55, 95% CI: 0.28-1.07; p=0.08) (Figure 2). A total of 6 deaths (9.0%) occurred in the TMVR patients vs 9 (12.3%) in their GDMT counterparts (HR 0.71, 95% CI: 0.25-1.99; p=0.47) (Figure 3). Eleven patients (16.4%) in the TMVR group had at least one unplanned HF hospitalisation, as compared with 22 patients (30.1%) in the GDMT group (HR 0.47, 95% CI: 0.23-0.97; p=0.04) (Figure 4). Last, major adverse cardiac events occurred in 16 (23.9%) patients in the TMVR group compared with 24 (32.9%) in the GDMT group (HR 0.54, 95% CI: 0.27-1.05; p=0.07). The sensitivity analysis found very comparable results, since the predicted cumulative rate of HF hospitalisation was 26 events per 100 patient-years in the TMVR group as compared with 51 events per 100 patient-years in the GDMT group (HR 0.44, 95% CI: 0.19-0.98; p=0.045). The HR for the cumulative rate of HF hospitalisation was 1.13 (95% CI: 0.81-1.56) between 0 and 12 months vs 0.46 (95% CI: 0.22-0.98; p=0.044) between 12 and 24 months after inclusion in the MITRA-FR trial in the “cause-specific hazard" model (pinteraction=0.032). Similarly, the HR for the cumulative rate of HF hospitalisation was 1.09 (95% CI: 0.79-1.51) between 0 and 12 months vs 0.44 (95% CI: 0.21-0.93; p=0.03) between 12 and 24 months after inclusion in the MITRA-FR trial in the Fine-Gray model (pinteraction=0.029).
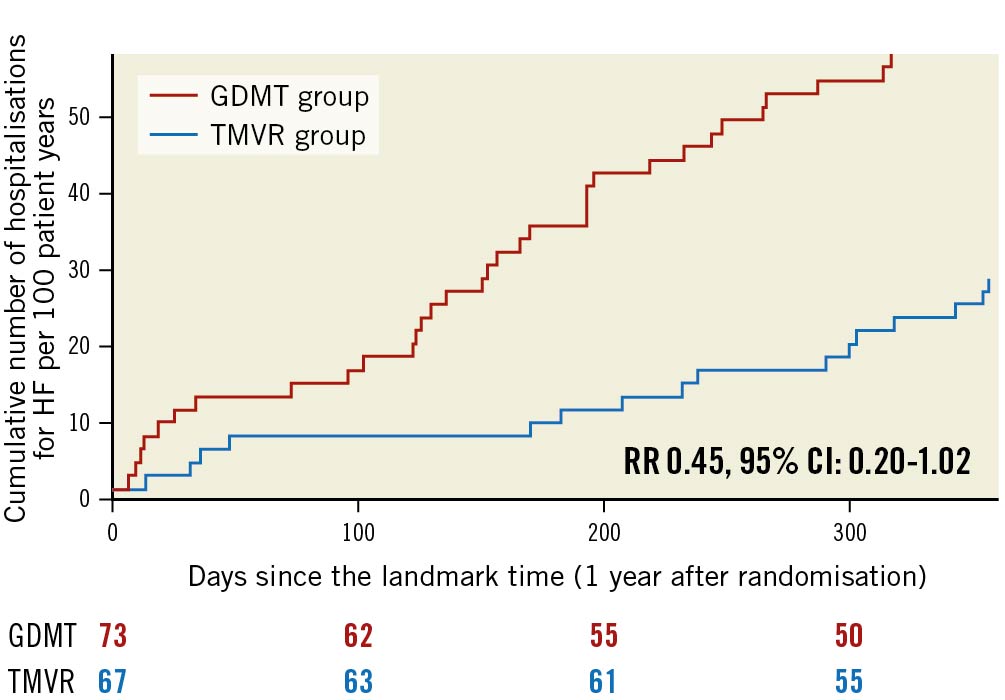
Figure 1. Cumulative rates of recurrent hospitalisations for heart failure in the guideline-directed medical treatment (GDMT) and in the transcatheter mitral valve repair (TMVR) groups between 12 and 24 months after inclusion in the MITRA-FR trial. CI: confidence interval; HF: heart failure; RR: rate ratio
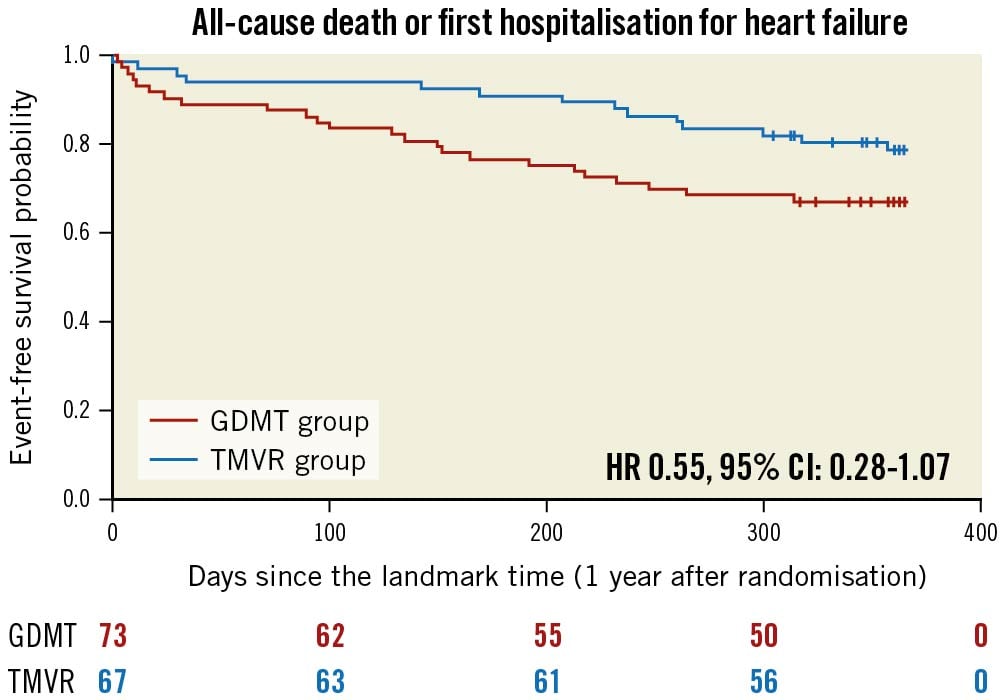
Figure 2. Kaplan-Meier estimates for all-cause death or unplanned heart failure hospitalisation in the guideline-directed medical treatment (GDMT) and in the transcatheter mitral valve repair (TMVR) groups between 12 and 24 months after inclusion in the MITRA-FR trial. CI: confidence interval; HR: hazard ratio
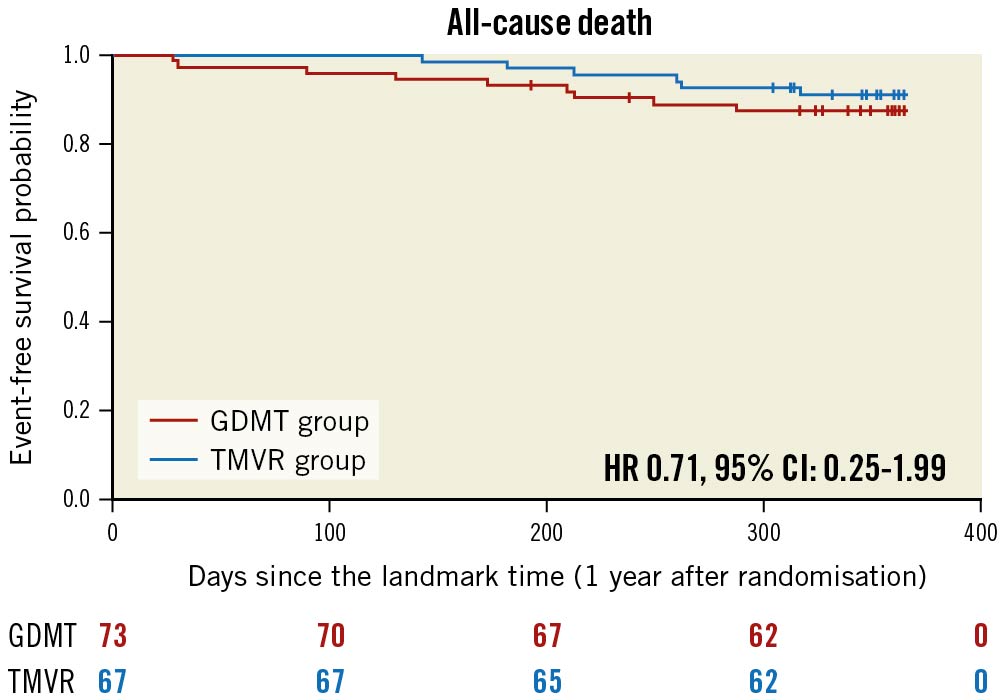
Figure 3. Kaplan-Meier estimates for all-cause death in guideline-directed medical treatment (GDMT) and in the transcatheter mitral valve repair (TMVR) groups between 12 and 24 months after inclusion in the MITRA-FR trial. CI: confidence interval; HR: hazard ratio
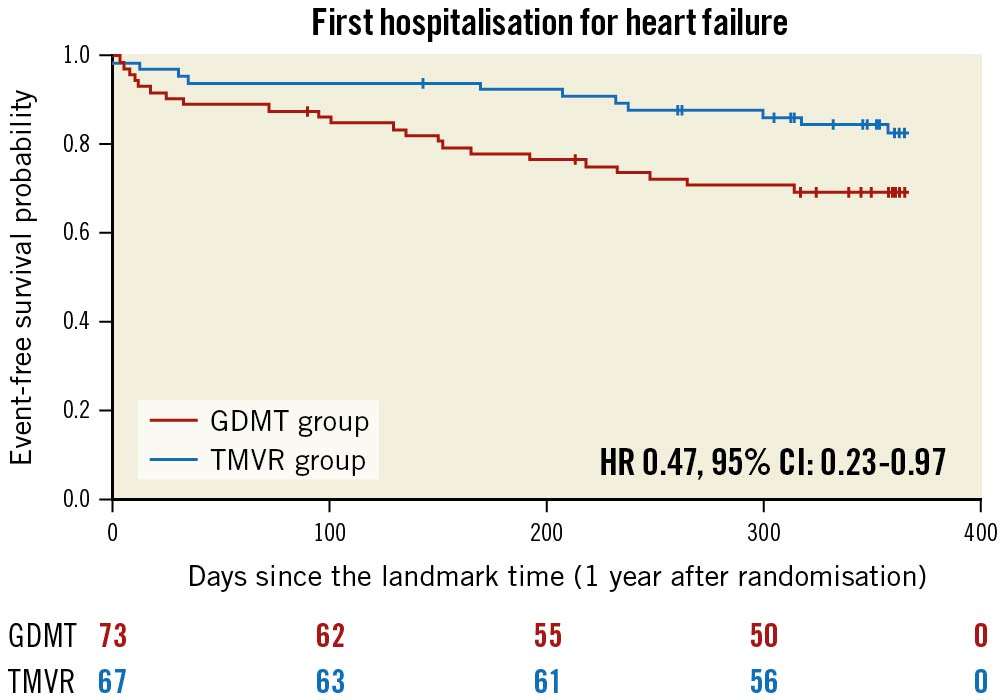
Figure 4. Kaplan-Meier estimates for unplanned heart failure hospitalisation in the guideline-directed medical treatment (GDMT) and in the transcatheter mitral valve repair (TMVR) groups between 12 and 24 months after inclusion in the MITRA-FR trial. HR: hazard ratio; CI: confidence interval
Landmark analysis selecting all eligible patients
The results regarding the analysis selecting all eligible patients (i.e., all the 12-month surviving MITRA-FR patients, whether they were hospitalised or not during the first year of follow-up) are extensively presented in Supplementary Table 2 and Supplementary Table 3. This analysis included 226 patients (TMVR group: 113; GDMT group: 113) and found very comparable results to the analysis including only surviving patients who were free from HF hospitalisation within the first 12 months of follow-up. Thus, characteristics in the TMVR and GDMT groups were similar at inclusion in the MITRA-FR trial. In addition, the number of patients hospitalised for HF during the first year was similar in the TMVR and GDMT groups (35.4% vs 40.7%; p=0.49). Selected patients presented with less severe characteristics at inclusion in the MITRA-FR trial and were less frequently hospitalised for HF during the first year (38.1% vs 76.9%; p<0.0001) than the patients who died during the first year. Interestingly, the primary outcome reached statistical significance: the predicted cumulative rate of HF hospitalisation was 38 events per 100 patient-years in the TMVR group as compared with 69 events per 100 patient-years in the GDMT group (HR 0.55, 95% CI: 0.31-0.98; p=0.042) (Supplementary Figure 1 - Supplementary Figure 4).
Discussion
The main findings of this 12-month landmark analysis of patients included in the MITRA-FR trial are 1) the patients who reached the primary endpoint within the first year after randomisation presented with more severe characteristics than those who didn’t and 2) despite similar characteristics at inclusion in the trial, the patients randomised to the intervention group who did not reach the trial’s primary endpoint during the first year underwent almost half as many accumulated hospitalisations for HF during the second year following inclusion than patients treated with GDMT alone (Central illustration).
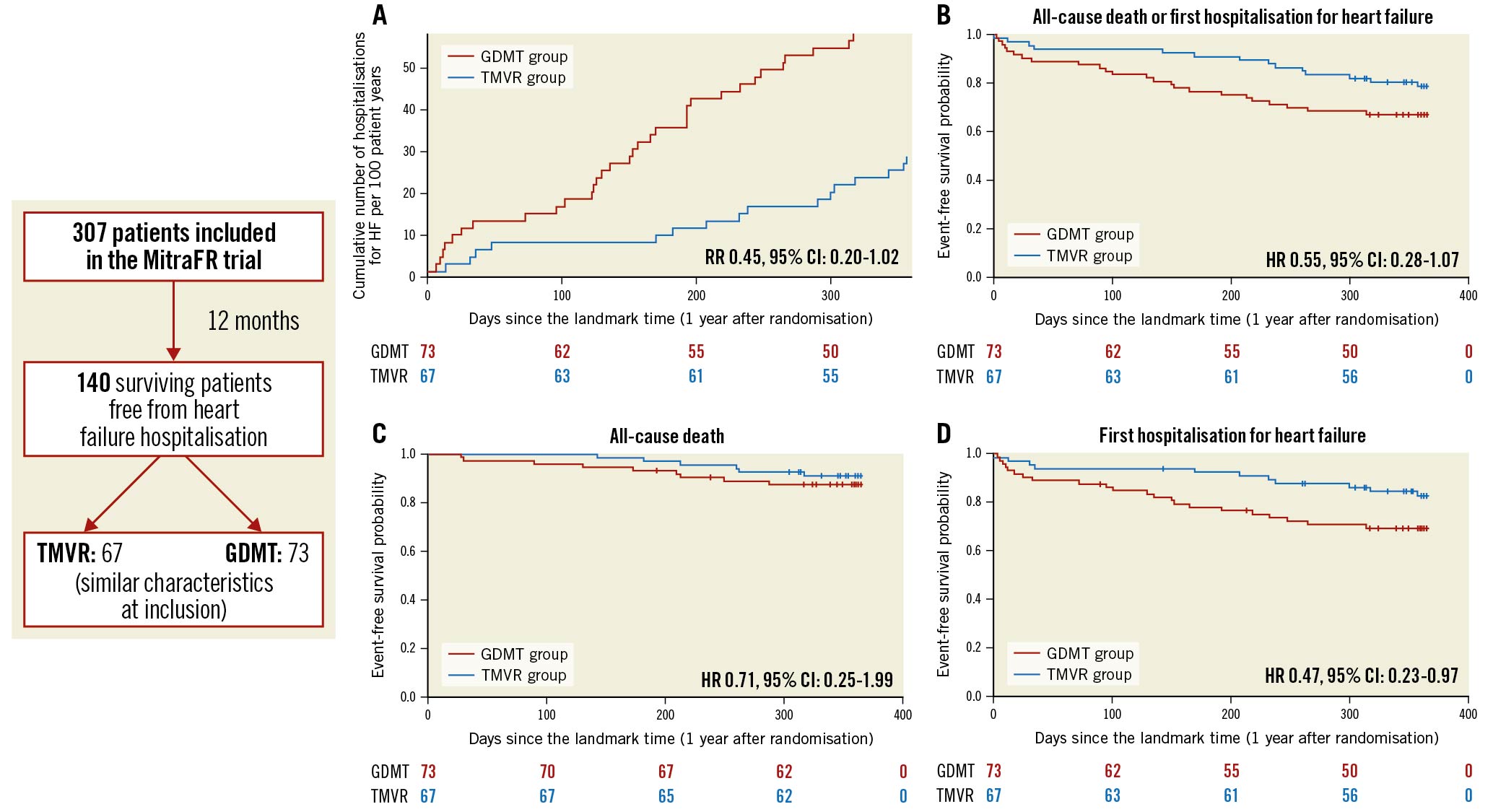
Central illustration. Main outcomes of this landmark analysis of the MITRA-FR trial. Cumulative rates of recurrent hospitalisations for heart failure (A), and Kaplan-Meier estimates for secondary outcomes in the guideline-directed medical treatment (GDMT) and in the transcatheter mitral valve repair (TMVR) groups between 12 and 24 months after inclusion in the MITRA-FR trial: all-cause death or unplanned heart failure hospitalisation (B), all-cause death (C), and hospitalisation for heart failure (D). CI: confidence interval; HR: hazard ratio; RR: rate ratio
Both the COAPT and MITRA-FR trials studied patients with severe HF. However, inclusion criteria for the MITRA-FR study selected patients with SMR mainly related to the severity of the LV dilatation than to a predominant valvular component. The prognosis of these patients is known to be particularly dire and the MITRA-FR study results showed a very high mortality rate during the first 12 months: 24.3% in the intervention group and 22.4% in the control group, associated with a high rate of unplanned hospitalisation for HF amounting to approximately 50% in both groups1. However, a two-year analysis of the MITRA-FR study showed stable and comparable mortality in both groups after 1 year of follow-up.
Potential delayed reduction of HF hospitalisation rate
While the COAPT trial found a substantial reduction of the hospitalisation rate within 24 months (HR 0.53, 95% CI: 0.40-0.70) in patients receiving TMVR in addition to GDMT compared with patients receiving GDMT alone, the MITRA-FR trial showed no difference between the 2 groups for its primary and secondary endpoints, especially for the time to first HF hospitalisation at 12 and 24 months. It is likely that the first HF admission and total HF admissions are linked in most of the diseases. However, it might be hypothesised that the hospitalisation rate, representing the overall HF burden of these patients, is a more relevant criterion, allowing a better characterisation and quantification of the patient’s journey throughout the follow-up period, than first HF hospitalisation12.
Moreover, the population of this landmark analysis harboured major differences compared with the patients included in the MITRA-FR trial who died or were hospitalised for HF within the first year of inclusion. The patients included in the landmark analysis presented with less severe characteristics than the excluded patients, with fewer comorbidities (including less renal failure), lower risk scores and better systolic left ventricular function. In other words, the most severely affected patients from the overall MITRA-FR trial population were not included in this landmark analysis. Therefore, it might be hypothesised that the landmark analysis patients had less advanced disease than their non-included counterparts. Besides, renal function is an important parameter to consider, as well as comorbidities, which have a significant impact on the prognosis of these patients with severe SMR.
The identification of predictors of short-term poor prognosis in patients with HF and severe SMR were reported in several publications, mainly based on large TMVR registries131415. Also, recent analyses from the COAPT trial highlighted interesting baseline elements associated with a worse prognosis in such patients, regardless of their management (TMVR or not), such as elevated PASP (≥50 mmHg)16, functional status assessed by the 6-min walk distance17, more than moderate tricuspid regurgitation18, and NYHA Functional Class >II19.
However, prediction of a mid-term positive response to mitral valve (MV) repair is still unclear. In the MITRA-FR trial, there were no significant interactions between trial groups and any of the prespecified subgroups with respect to the rate of the primary outcome event at 12 and 24 months. A post hoc MITRA-FR study focusing on multiple echocardiographic parameters failed to identify specific subgroups who might benefit from MV repair20. Furthermore, an in-depth analysis of the MITRA-FR data recently highlighted that the 2-year outcome of its COAPT-eligible population was similar in the intervention and control groups21. Thus, a COAPT-like pattern itself did not warrant a favourable outcome. Moreover, a recent analysis of the COAPT trial found no reduction in the composite of all-cause mortality or HF hospitalisations at 24 months with TMVR among patients with a MITRA-FR pattern22.
A previous study on patients with HF and severe SMR on GDMT alone highlighted a significant association between a low EROA/LVEDV ratio and a better outcome23. Similarly, in a recently published report, patients on GDMT alone with significant SMR and a regurgitant volume to LVEDV ratio ≥20% tended to have higher mortality rates than those with a ratio <20%24. Actually, the EROA/LVEDV ratio was lower in the MITRA-FR patients analysed in this landmark analysis than in the non-selected patients. It has been suggested that this ratio, by taking into account the severity of the MR (shown by the EROA) and the LV remodelling (LVEDV), may stratify patients. According to this proportionate/disproportionate SMR framework, both landmark-included and non-included patients should, however, be considered as patients with “proportionate SMR” (SMR-LV-codominant)89.
Other parameters, such as the right ventricular function, LV fibrosis and LV contractile reserve, are possibly different between the two trials’ populations and might also explain the observed divergent outcomes. This landmark analysis supports this hypothesis. Unidentified factors may explain the discrepancy between the two studies. Future analysis of the pooled individual data of the COAPT and MITRA-FR trials may highlight specific and strong predictors of good responders to MV repair.
Landmark analysis
A landmark analysis is an observational method used to compare time-to-event outcomes between groups determined during study follow-up. It aims to estimate the time-to-event probabilities in each group according to the group membership of patients at a specific time point, the landmark time. Despite being a valuable methodological analysis method, landmark analysis has several major limitations25. First, because of the lack of randomisation in the constitution of the groups, this work remains an observational analysis, with the known limitations inherent to this design. However, it is worth noting that the two groups (TMVR and GDMT alone) had similar characteristics at inclusion in the MITRA-FR trial, except for history of myocardial infarction, which was more frequent in the device group, this difference was present at baseline as well as in the landmark analysis. Prognosis of ischaemic HF is expected to be worse than idiopathic cardiomyopathy-related HF26.
Moreover, the arbitrary selection of the landmark time is a well-known limit of this method. For this work, we hypothesised that a 12-month delay is long enough for the distinction of the most seriously affected patients. In addition, 12 months is a commonly accepted threshold for assessment of a potential benefit of any procedure. Accordingly, the Mitral Valve Academic Research Consortium (MVARC) patient success is measured at 12 months.
Limitations
Aside from the previously discussed limits of a landmark analysis, this was a post hoc analysis of a non-prespecified outcome in the setting of a negative randomised trial. As such, our results should be regarded as hypothesis-generating. We considered the annualised HF hospitalisation rate as the primary outcome for this work, which is essentially aligned with the COAPT trial. Moreover, because a significant amount of data (mainly echocardiographic) were missing at 12 months after inclusion in the MITRA-FR trial, we were not able to compare patients’ characteristics in the intervention and control groups at the landmark point. It is very likely because of survivorship bias that patients reaching the landmark point were overall healthier than patients who died or were hospitalised during the first 12 months. Furthermore, the lack of information about the potential uptitration of the medical therapies within the first year of follow-up impeded the analysis of the exact role of GDMT in both groups. Besides, this analysis failed to identify clear predictive factors, such as the dilatation of the LV, associated with a favourable outcome. The absence of a predictive model to identify those patients who would remain event-free at 1-year follow-up reduces the insights from this study in clinical practice. This analysis included a limited number of patients, since the rate of MITRA-FR primary endpoints at 12 months was high in the overall population. Thus, the absolute number of events within the studied period was quite small, which reduced the statistical power of the analysis. The risk of a type II error (false negative) cannot be ruled out to explain the absence of statistical significance in the analysis of the primary outcome despite an absolute cumulative rate of HF hospitalisation, which was almost twice those of the TMVR group in the GDMT group. In keeping with this point, we were not able to analyse the primary outcome using a joint frailty model accounting for the competitive risk of death, which was the methodology of the COAPT trial, because of the limited number of events. Last, it should be stressed that randomisation is lost when analysing patients at the landmark point. Similar baseline characteristics among the two groups do not reduce the risk of residual confounding (i.e., unmeasured characteristics) in the analyses.
Conclusions
This 12-month landmark analysis of the MITRA-FR trial showed an almost 2-fold lower annualised HF hospitalisation rate between 12 and 24 months in patients treated with TMVR compared with patients treated with GDMT alone, which did not reach statistical significance in the setting of a limited number of events. As in any exploratory analysis, these results should be viewed cautiously and regarded as hypothesis-generating. Further studies are required to confirm the potential ability of TMVR to improve the prognosis of severe symptomatic SMR.
Impact on daily practice
From the initial cohort of patients included in the MITRA-FR trial, those alive and free from HF hospitalisation at 1 year have a lower rate of HF hospitalisation within the 2 years of follow-up after randomisation, a difference that did not reach statistical significance. Future research is needed to identify the patients who stand to benefit the most long-term from TMVR for severe and symptomatic secondary MR.
Funding
The MITRA-FR trial was financed by an academic grant from the French Ministry of Health.
Conflict of interest statement
G. Leurent reports proctoring activity and received lecture and consultant fees from Abbott. V. Auffret received lecture fees from Medtronic and BMS/Pfizer. E. Donal received research grants from General Electric and Abbott. G. Bonnet received proctoring fees from Abbott. P.Y. Leroux reports proctoring fees from Abbott. P. Guerin reports proctoring fees from Abbott; received research grants from Abbott and Edwards Lifesciences. T. Lefèvre received proctoring fees from Abbott. D. Messika-Zeitoun received research grants from Edwards Lifesciences. B. Iung received lecture fees from Edwards Lifesciences. J.N. Trochu received institutional grants from Boston Scientific and Novartis and personal/consulting fees from Abbott, Bayer, Novartis, Boston Scientific and Vifor Pharma. J.F. Obadia received consultant fees from Abbott, Carmat, Delacroix-Chevalier, Medtronic and Landanger. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

