
In these pages, we recently postulated whether the results of the MITRA-FR study signalled either the end of the road or simply a speed bump in the use of transcatheter valve repair with the MitraClip® device (Abbott Vascular, Santa Clara, CA, USA) to treat functional mitral regurgitation (FMR) in high-risk patients with symptomatic heart failure1. The results of the Cardiovascular Outcomes Assessment of the MitraClip Percutaneous Therapy for Heart Failure Patients with Functional Mitral Regurgitation (COAPT) trial presented with great fanfare on 23 September at TCT 2018 in San Diego, USA, and simultaneously published in the New England Journal of Medicine, clearly suggest the latter2.
The COAPT study enrolled patients with moderate-to-severe or severe FMR who remained in NYHA Class II-IV despite guideline-directed medical therapy (GDMT) to MitraClip plus GDMT or GDMT alone. Patients were required to have left ventricle ejection fraction (LVEF) 20-50%, left ventricle end-systolic diameter ≤70 mm, and either a heart failure hospitalisation in the previous 12 months or elevated NT-proBNP ≥1,500 pg/ml. The primary effectiveness endpoint, powered for superiority, was a reduction in heart failure hospitalisation at two years. Various important secondary endpoints were also powered for superiority of the device arm, including MR ≤2+, all-cause mortality at 12 months, death and heart failure hospitalisation up to 24 months, as well as quality of life, six-minute walk distance, and NYHA Class I/II at 12 months. The primary safety endpoint included a variety of procedural and device-related complications at one year compared to an historical objective performance goal.
Among 1,576 patients submitted to the clinical events committee (CEC) for study inclusion, 911 (57.8%) were not enrolled, principally since they did not satisfy the stringent echocardiographic inclusion criteria. Among 78 study sites, 34 without extensive/recent MitraClip experience treated 51 roll-in cases, and 614 patients were subsequently randomised to the MitraClip (n=302) or medical therapy (312) groups. Included patients were on average 72 years old and had features of an advanced heart failure population – 55% in atrial fibrillation, >40% with STS score ≥8%, >60% in NYHA Class III/IV, 36% with prior cardiac resynchronisation therapy. Mitral regurgitation was severe (4+) in 50% of cases, the average estimated regurgitant orifice area (EROA) was 0.41 cm2 and left ventricular ejection fraction was 31%. GDMT was excellent in both groups at baseline and between-group differences did not emerge during follow-up. Among those assigned to MitraClip, 95% had at least one clip implanted, the mean number of clips used was 1.7 per patient, and echocardiography at hospital discharge revealed that 17.7% had MR ≥grade 2. At two years, the primary effectiveness endpoint hospitalisation was considerably reduced in the MitraClip arm compared to medical therapy (HR 0.53, 95% CI: 0.40-0.70, p<0.001) (Figure 1). To prevent one heart failure hospitalisation at two years, three patients (95% CI: 1.9-8.2) would require treatment with the MitraClip (number needed to treat [NNT]). All of the powered secondary endpoints favoured treatment with the MitraClip, most notably all-cause mortality at two years, where an impressive absolute risk reduction of 17% was achieved (HR 0.62, 95% CI: 0.46-0.82, p<0.001). The NNT for all-cause mortality was 5.9 (95% CI: 3.9-11.7). The primary safety endpoint easily surpassed the historical objective performance goal.
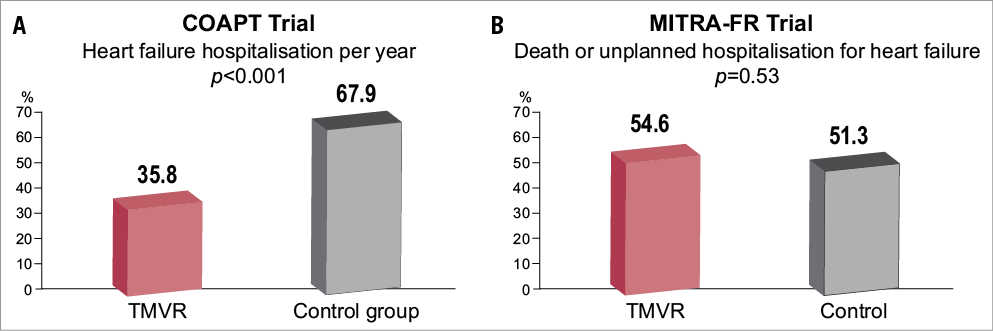
Figure 1. COAPT vs MITRA-FR. A) Transcatheter mitral valve repair in patients with heart failure: COAPT Trial2. B) Transcatheter mitral valve repair versus medical treatment for secondary mitral regurgitation: MITRA-FR3. Details are published online at: https://www.pcronline.com/eurointervention/trials-2017/randomised-trials/
So, why are the results of COAPT so different from MITRA-FR? First, patients in COAPT were different: they had more severe FMR (EROA: COAPT 0.41±15 vs. MITRA-FR 0.31±10 cm2) and, despite similar LVEF, had less dilated LVs (LV end-diastolic volume [LVEDV]: COAPT 101±34 vs. MITRA-FR 135±35 mL/m2)3. Second, the acute and longer-term outcome of the MitraClip procedure was superior in COAPT: procedural complications (MITRA-FR definition) occurred in 8.5% and 14.6% in COAPT and MITRA-FR, respectively, acute post-procedural MR grade ≥3+ was reported in 5% in COAPT and 9% in MITRA-FR, and at one year MR grade ≥3 was evident in 5% in COAPT and 17% in MITRA-FR.
Taken together, these two studies have considerable implications for our patients and our hospitals, for the specialities of heart failure and cardiovascular surgery, and for ongoing research and development in the mitral space. COAPT has rendered invalid the hypothesis that FMR is solely a disease of the left ventricle. Left ventricular dysfunction and dilatation initiate FMR but, as MR progresses, it begets further MR, LV failure, and impacts on prognosis. The reduction in FMR with the MitraClip is likely the mechanism for the reported improvement in prognosis, quality of life, and functional capacity. However, the results of MITRA-FR and COAPT also indicate that not all patients with FMR may benefit from MitraClip; patients with considerably dilated left ventricles and less mitral regurgitation may not improve with this therapy.
It is likely that other transcatheter therapies for FMR, such as transcatheter mitral annuloplasty with the Edwards Cardioband (Edwards Lifesciences, Irvine, CA, USA), can have a similar impact to the MitraClip in appropriately selected patients, if they can effectively reduce MR with similarly low procedural complications. Mitral valve surgery effectively abolishes FMR but the high rates of procedural complications have yielded a neutral impact on long-term prognosis4.
COAPT will necessitate modification of the European valvular heart disease guidelines5. In selected symptomatic FMR patients on optimal medical therapy, treatment allocation to either mitral valve repair surgery (Class IIb/LOE C) or MitraClip (Class IIb/LOE C) is determined by surgical risk. One could envisage that MitraClip will become a Class IIa recommendation, irrespective of surgical risk, while mitral valve surgery continues as Class IIb. The impact of this change will have important implications for the delivery of patient care. Already, hospitals have had to accommodate the development of transcatheter aortic valve implantation as the dominant strategy for aortic stenosis. The rollout of transcatheter mitral valve repair will have significant implications for healthcare resource allocation, service development planning, assessment of equitable patient access, and physician training. Moreover, there may be a negative impact on institutional volume of heart valve surgeries.
The results of COAPT will advance the development of other heart valve therapies for both functional and degenerative MR. Transcatheter mitral valve replacement (TMVR), in particular, will accelerate as these devices have a greater capacity to abolish MR, though currently at a cost of much higher procedural mortality6. Current and future trial designs testing novel solutions in the mitral space will also have to consider the MitraClip as the control arm rather than optimal medical therapy. Indeed, recruitment into ongoing trials of transcatheter repair and replacement devices will be more difficult given the results of COAPT. Clinicians will be rightly hesitant to randomise their patients to a novel treatment if they are candidates for the MitraClip.
One other important trial of MitraClip in FMR and heart failure remains outstanding. The Randomized Study of the MitraClip Device in Heart Failure Patients With Clinically Significant Functional Mitral Regurgitation (RESHAPE-HF) trial (NCT01772108) has randomised 800 patients to MitraClip and optimal medical therapy or medical therapy alone. Will this study ultimately decide the fate of MitraClip in FMR or has the die already been cast on the results of COAPT? The impressive reduction in heart failure mortality observed in COAPT offers great hope to a high-risk patient group with few therapeutic alternatives. If the spontaneous applause witnessed during the presentation of the COAPT results at TCT 2018 can be considered a surrogate for the potential impact of an interventional treatment, then edge-to-edge mitral valve repair with the MitraClip will have a defining impact on our speciality.

