Left ventricular ejection fraction (LVEF) is the foundation of current classification for patients with heart failure (HF)1. It can be easily measured by echocardiography, has a close relation with outcomes and defines those patients with an indication to current evidence-based treatment for HF1,2,3.
However, many shortcomings of LVEF have been shown recently. Measurement of LVEF is operator-dependent with high inter- and intra-observer variability and is influenced by geometrical assumptions that make volume estimation inaccurate when based on two-dimensional images, such as with echocardiography. Second, the severity of the impairment in longitudinal and circumferential myocardial fibre layers may differ and make LVEF relatively insensitive to detect abnormalities in the early stages of HF as compared with other measurements, such as LV global longitudinal strain3. Lastly, LVEF is both preload- and afterload-dependent and is influenced by changes in loading conditions and LV structure and geometry such as LV remodelling, hypertrophy, and dyssynchrony3.
Among the many variables that influence LVEF, mitral regurgitation (MR) is one of the most important3. Reduction in LV afterload by MR and the inclusion of the regurgitant volume in the stroke volume calculation leads to an overestimation of LV systolic function when LVEF is used in patients with MR. However, LVEF still retains a major role and is a major inclusion criterion for clinical trials in patients with HF and MR4,5,6,7,8.
In this issue of EuroIntervention, Lerakis et al report the impact of LVEF in COAPT (Cardiovascular Outcomes Assessment of the MitraClip Percutaneous Therapy for HF patients with functional MR)9.
In this trial, LVEF was not an independent predictor of the composite endpoint of death or HF hospitalisations both when analysed as a continuous variable and when dichotomised at a cut-off value of 40%. When patients were subdivided with a cut-off value of 30%, those with an LVEF <30% had a higher rate of the composite endpoint and of HF hospitalisations alone, compared to those with an LVEF >30% though, again, with no difference regarding mortality alone and changes in quality of life (QOL) and the six-minute walking test distance (6MWTD)9.
Second, and more importantly, the present analysis shows that the impact of percutaneous edge-to-edge mitral valve repair in reducing death and HF hospitalisations, both as a combined endpoint and individually, as well as in improving MR severity, QOL and 6MWTD, was independent from LVEF values, regardless of the cut-off values used for stratification and with similar results when LVEF was analysed as a continuous variable9. Thus, even patients with preserved LV function or, at the other end of the spectrum, with severe LV dysfunction had a better outcome when treated percutaneously in COAPT9.
These results from COAPT partially differ from other studies. First, an observational study including patients with HF on optimal medical therapy showed that secondary MR was associated with poor outcome in patients with an LVEF between 30% and 40%, but not in those with an LVEF <30%, suggesting that secondary MR may not be a therapeutic target in these patients with more severe LV dysfunction5. Second, although the results were also independent from LVEF in this trial, percutaneous treatment had no effect on outcomes in MITRA-FR (Multicentre Study of Percutaneous Mitral Valve Repair MitraClip Device in Patients With Severe Secondary Mitral Regurgitation)6,7.
How can the lack of impact of LVEF on patients’ outcomes and on the favourable results of percutaneous treatment of MR in COAPT be explained? Careful patient selection, and possibly better outcomes of the percutaneous procedure were probably the key elements of the success of COAPT. Some aspects can already be identified looking at the LVEF inclusion criteria. MITRA-FR included only patients with an LVEF <40% with no lower limitation. In contrast, COAPT allowed patients with an LVEF of up to 50% and, actually, 18% of their patients had an LVEF of between 40% and 50%, and also excluded patients at the lowest end of the spectrum, with an LVEF <20%8. However, other enrolment criteria could have been crucial. COAPT excluded patients with an LV end-systolic diameter >70 mm, non-ambulatory New York Heart Association (NYHA) Class IV symptoms or haemodynamic instability, evidence of right ventricular dysfunction, severe pulmonary hypertension or severe tricuspid regurgitation. This resulted in a rather slow and very careful enrolment into the trial. These criteria probably ensured the success of percutaneous treatment independently from the LVEF at baseline.
Analyses of a real-world setting cohort recently confirmed the value of the COAPT criteria. Patients who fulfilled these criteria, namely lack of haemodynamic instability, lack of severe LV impairment and lack of right ventricular dysfunction and/or severe tricuspid regurgitation and/or severe pulmonary hypertension, had better outcomes after percutaneous treatment of MR compared with those who had one or more of them10. Interestingly, while the Kaplan-Meier curves of the patients with haemodynamic instability or right ventricular dysfunction or severe pulmonary hypertension, compared with the others, started to diverge early during follow-up, those of the patients with severe LV impairment started to diverge much later, i.e., after two years, thus showing a different role of LV impairment, occurring at a later stage after the percutaneous procedure10.
Thus, Lerakis et al must be praised for their careful analysis of COAPT. They showed the limitations of LVEF as a prognostic marker and as a predictor of the beneficial effects of percutaneous treatment of MR. However, these results must be considered in the light of the careful selection criteria that were used in COAPT. Lerakis et al also showed the value of taking into account other patient characteristics, in addition to LVEF, including LV structure, geometry and dimensions, right ventri-cular function, pulmonary hypertension, concomitant valve disease and, last but not least, clinical stability and optimal medical treatment. A multiparametric approach, including all the features reported above, may guarantee the success of percutaneous treatment of MR (Figure 1).
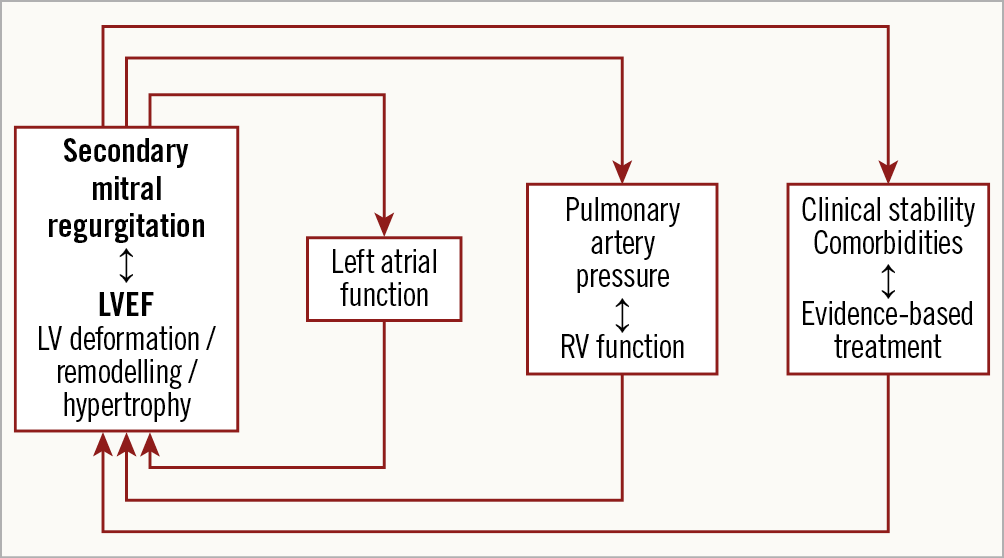
Figure 1. Multiparametric approach for patients with HF and MR.
Conflict of interest statement
M. Metra reports personal fees from Actelion, Amgen, AstraZeneca, Abbott Vascular, Bayer, Servier, Edwards Therapeutics, LivaNova, Vifor Pharma, Windtree Therapeutics, as member of trials' committees or for speeches at sponsored meetings. M. Adamo has no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

