Abstract
Background: In the COAPT trial, transcatheter mitral valve repair with the MitraClip plus maximally tolerated guideline-directed medical therapy (GDMT) improved clinical outcomes compared with GDMT alone in symptomatic patients with heart failure (HF) and 3+ or 4+ secondary mitral regurgitation (SMR) due to left ventricular (LV) dysfunction.
Aims: In this COAPT substudy, we sought to evaluate two-year outcomes in HF patients with reduced LV ejection fraction (HFrEF; LVEF ≤40%) versus preserved LVEF (HFpEF; LVEF >40%) and in those with severe (LVEF ≤30%) versus moderate (LVEF >30%) LV dysfunction.
Methods: The principal effectiveness outcome was the two-year rate of death from any cause or HF hospitalisations (HFH). Subgroup analysis with interaction testing was performed according to baseline LVEF; 472 patients (82.1%) had HFrEF (mean LVEF 28.0%±6.2%; range 12% to 40%) and 103 (17.9%) had HFpEF (mean LVEF 46.6%±4.9%; range 41% to 65%), while 292 (50.7%) had severely depressed LVEF (LVEF ≤30%; mean LVEF 23.9%±3.8%) and 283 (49.3%) had moderately depressed LVEF (LVEF >30%; mean LVEF 39.0%±6.8%).
Results: The two-year rate of death or HFH was 56.7% in patients with HFrEF and 53.4% with HFpEF (HR 1.16, 95% CI: 0.86-1.57, p=0.32). MitraClip reduced the two-year rate of death or HFH in patients with HFrEF (HR 0.50, 95% CI: 0.39-0.65) and HFpEF (HR 0.60, 95% CI: 0.35-1.05), pint=0.55. MitraClip was consistently effective in reducing the individual endpoints of mortality and HFH, improving MR severity, quality of life, and six-minute walk distance in patients with HFrEF, HFpEF, LVEF ≤30%, and LVEF >30%.
Conclusions: In the COAPT trial, among patients with HF and 3+ or 4+ SMR who remained symptomatic despite maximally tolerated GDMT, the MitraClip was consistently effective in improving survival and health status in patients with severe and moderate LV dysfunction and those with preserved LVEF.
Introduction
The mitral valve is a dynamic and complex structure1,2. In patients with heart failure (HF), geometric displacement of the papillary muscles and chordae may result in leaflet tethering and lack of coaptation of the mitral leaflets, with secondary (or functional) mitral regurgitation (SMR)3. In SMR, disruption of mitral valve function is caused principally by the underlying left ventricular (LV) dilatation rather than structural derangements in the mitral valve complex per se4. As a result, guideline-directed medical therapy (GDMT) for LV dysfunction is the foundation of the management of SMR5,6. Substantial SMR is associated with decreased quality of life (QOL), increased rates of hospitalisation for HF, and diminished survival7,8. GDMT and cardiac resynchronisation therapy in selected patients have been shown to reduce the severity of SMR and provide symptomatic relief7.
With the advent of transcatheter mitral valve repair (TMVr), treatment options for SMR have expanded9,10,11. Two landmark randomised trials have recently evaluated the role of transcatheter leaflet approximation with the MitraClip™ (Abbott, Santa Clara, CA, USA) in HF patients with SMR. In the COAPT trial, TMVr with the MitraClip markedly improved two-year survival and health status compared with maximally tolerated GDMT alone in symptomatic patients with HF and moderate-to-severe (3+) or severe (4+) SMR due to LV dysfunction3,12. In contrast, in the MITRA-FR trial there were no significant differences at one year or two years in any of these endpoints1,13. Important differences in baseline patient characteristics, background therapies, and treatments may explain the discrepant outcomes of these trials5,6,12. Of note, although the mean LV ejection fractions (LVEF) were similar between the two studies, COAPT enrolled a substantial proportion of patients with HF and systolic dysfunction in the range often termed “preserved” EF (HFpEF; LVEF >40%), whereas enrolment in MITRA-FR was restricted to patients with HF and reduced EF (HFrEF; LVEF ≤40%). In the present study we sought to examine the outcomes from the COAPT trial according to LVEF to determine the extent to which the severity of LV dysfunction may impact on the potential benefits of TMVr in patients with HF and severe SMR.
Methods
TRIAL DESIGN
The rationale for and design of the COAPT trial have been published previously14. COAPT was a randomised, multicentre, controlled, parallel group, open-label trial of TMVr with the MitraClip in patients with HF and moderate-to-severe or severe SMR confirmed by an echocardiographic core laboratory. The protocol was planned according to the criteria dictated by the Mitral Valve Academic Research Consortium7,15. The study was approved by the institutional review board at each site, and each enrolled patient signed informed consent.
RANDOMISATION AND STUDY GROUPS
Patients with ischaemic or non-ischaemic cardiomyopathy with LVEF of 20% to 50% and moderate-to-severe (3+) or severe (4+) SMR who remained symptomatic despite maximally tolerated GDMT were eligible for enrolment. Detailed inclusion and exclusion criteria have been reported previously3,14. Details regarding randomisation, device implantation, and study procedures have been published previously14. Follow-up was performed at 1 week and 1, 6, 12, 18, and 24 months (the time of the primary endpoint) and is ongoing up to 5 years. Follow-up assessments included periodic echocardiography, six-minute walk distance (6MWD), and QOL measures, including the New York Heart Association (NYHA) functional class and Kansas City Cardiomyopathy Questionnaire (KCCQ). MitraClip treatment was not allowed in the control group until at or beyond the two-year follow-up time point.
OBJECTIVES AND ENDPOINTS
In the present study we sought to compare two-year outcomes of patients enrolled in the COAPT trial according to baseline LVEF. The principal endpoints for the COAPT trial have been described previously14. The primary effectiveness endpoint for the present study was the two-year composite rate of all-cause mortality or hospitalisations for HF, as adjudicated by an independent events committee after review of original source documents. We evaluated clinical and health status outcomes in patients with HFrEF (LVEF ≤40%) versus HFpEF (LVEF >40%) as well as in those with severe LV dysfunction (LVEF ≤30%) versus moderate LV dysfunction (LVEF >30%) at the time of enrolment. Of note, site-assessed LVEF was used as the criterion to qualify the patient for randomisation. For the present analysis, LVEF as assessed by the echocardiographic core laboratory on the baseline pre-randomisation transthoracic echocardiogram was used for LVEF stratification. As such, some patients were enrolled with an LVEF <20% or >50%.
STATISTICAL ANALYSIS
Categorical variables were compared with Fisher’s exact test, and continuous variables were compared with t-tests unless the data were not normally distributed, in which case the Wilcoxon rank-sum test was used. The times to the first occurrence of death or HF hospitalisation (HFH) were estimated using the Kaplan-Meier method and were compared with the log-rank test. Multivariable analysis was performed using Cox regression with LVEF forced into the model. Formal interaction testing was performed to determine whether there were differences in the hazard ratios of patients randomised to the device versus control groups according to LVEF subgroup. A spline analysis was also performed to evaluate the continuous relationship between baseline LVEF, randomisation arm, and the two-year rates and relative hazard of death or HFH. Changes in KCCQ and 6MWD from baseline to later intervals were performed by analysis of covariance, adjusting for baseline differences. All analyses were performed on an intention-to-treat basis. A two-sided p-value of <0.05 was considered statistically significant. Statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA).
Results
PATIENTS AND LVEF
From December 2012 to June 2017, 614 patients at 78 centres in the USA and Canada were enrolled in the trial. Baseline echocardiograms suitable for LVEF analysis by the echocardiographic core laboratory were available in 575 patients (94%), representing the current study population. By core laboratory analysis, the mean LVEF in the entire study population was 31.3%±9.3% (Figure 1); 472 patients (82.1%) had HFrEF (mean LVEF 28.0%±6.2%; range 12% to 40%) and 103 (17.9%) had HFpEF (mean LVEF 46.6%±4.9%; range 41% to 65%). LVEF was <20% and >50% in 32 and 13 patients, respectively. LVEF was severely depressed (LVEF ≤30%; mean LVEF 23.9%±3.8%) in 292 patients (50.7%) and moderately depressed (LVEF >30%; mean LVEF 39.0%±6.8%) in 283 (49.3%). Differences in baseline characteristics and medication use between patients with HFrEF versus HFpEF are shown in Supplementary Table 1 and Supplementary Table 2, while differences between patients with LVEF ≤30% versus >30% are shown in Supplementary Table 3 and Supplementary Table 4.
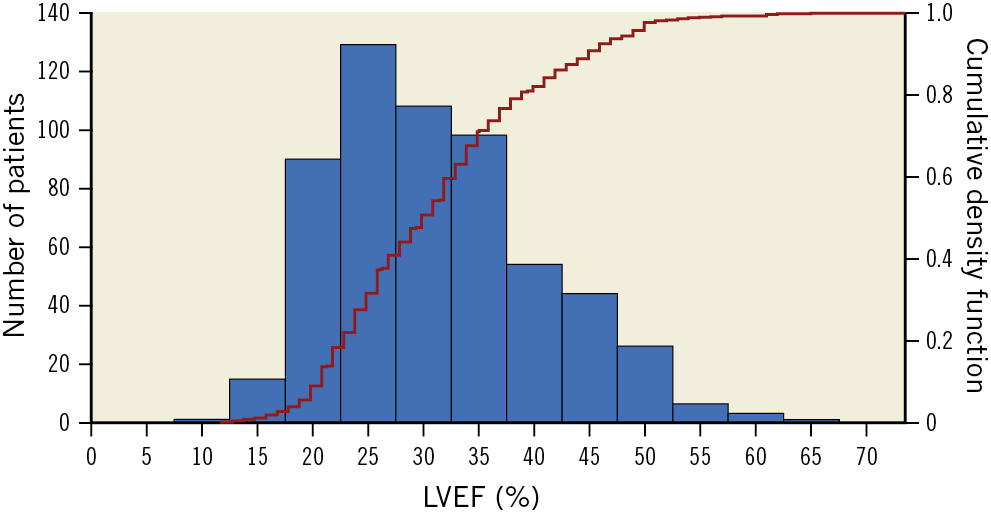
Figure 1. Histogram of left ventricular ejection fractions of patients enrolled in the COAPT trial. The left y-axis is the number of patients for each 5 percent increment of left ventricular ejection fraction (LVEF). The superimposed curve and corresponding right y-axis represent the cumulative frequency distribution of LVEF.
OUTCOMES ACCORDING TO LVEF
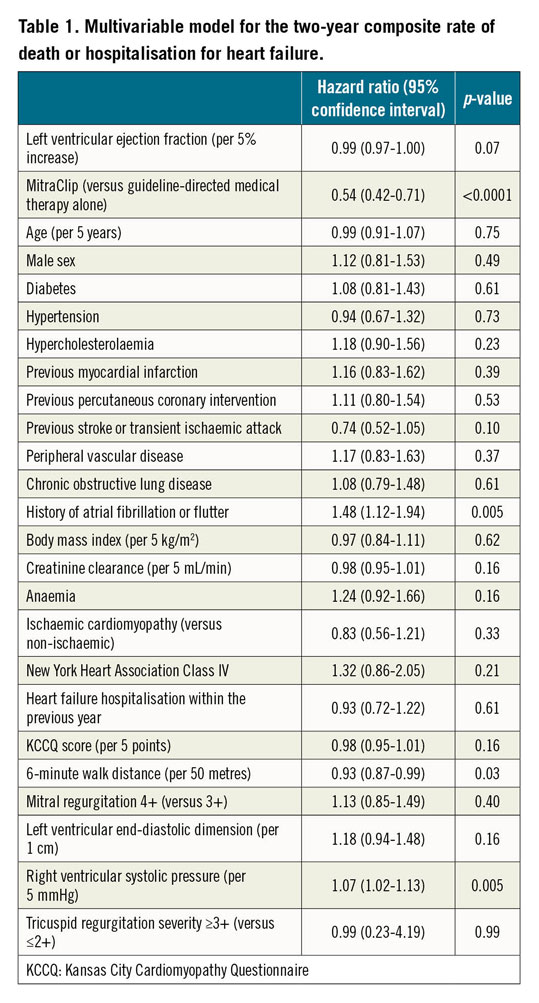
The composite endpoint of death or HFH occurred in 56.7% of patients with HFrEF and 53.4% with HFpEF (HR 1.16, 95% CI: 0.86-1.57, p=0.32) (Figure 2A). By multivariable analysis, LVEF (as a continuous variable) was not an independent predictor of the composite rate of death or HFH (adjusted HR per 5% increase in LVEF 0.99, 95% CI: 0.97-1.00, p=0.07) (Table 1). Similarly, there were no significant differences in the two-year individual rates of death or HFH (Figure 2B, Figure 2C) or improvement in KCCQ or 6MWD over time between HFrEF and HFpEF (Supplementary Table 5). The composite endpoint of death or HFH occurred in 61.3% of patients with LVEF ≤30% and in 50.6% with LVEF >30% (HR 1.3, 95% CI: 1.04, 1.62, p=0.02) (Supplementary Figure 1A). The individual endpoint of HFH occurred in 52.5% of patients with LVEF ≤30% and in 38.8% with LVEF >30% (HR 1.48, 95% CI: 1.14, 1.91, p=0.003) (Supplementary Figure 1B). There was no significant difference in the two-year rate of death or improvement in KCCQ or 6MWD over time between patients with LVEF ≤30% versus >30% (Supplementary Table 6, Supplementary Figure 1C).
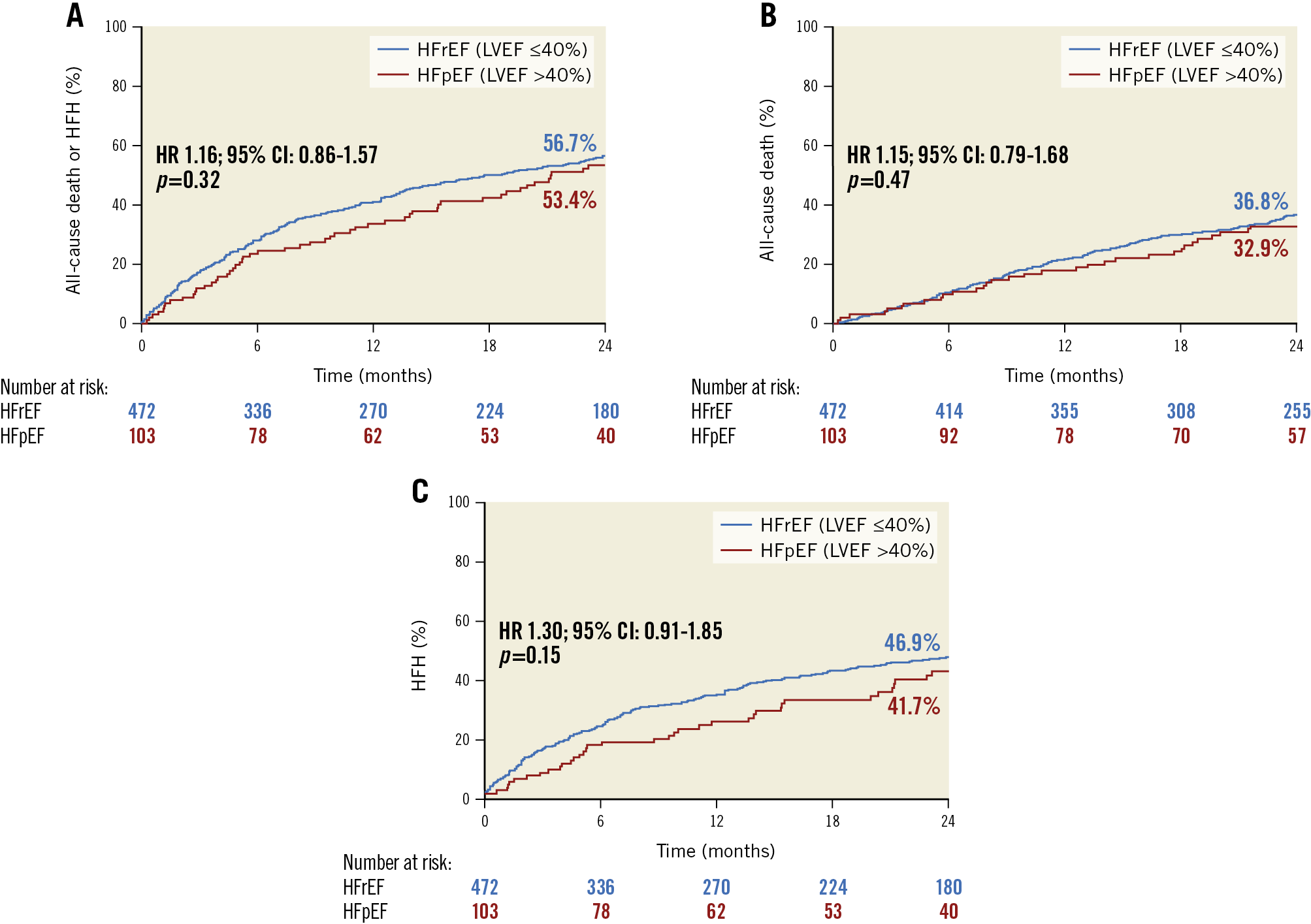
Figure 2. Kaplan-Meier time-to-first-event curves in patients with HFrEF versus HFpEF. A) Death or heart failure hospitalisation (HFH). B) Death. C) HFH. CI: confidence interval; HFpEF: heart failure with preserved ejection fraction; HFrEF: heart failure with reduced ejection fraction; HR: hazard ratio
IMPACT OF MITRACLIP TREATMENT ACCORDING TO LVEF
Among the 472 HFrEF patients, 231 were randomised to MitraClip plus GDMT and 241 to GDMT alone. Among the 103 HFpEF patients, 50 were randomised to MitraClip plus GDMT and 53 to GDMT alone. The baseline characteristics in both subgroups were well matched between the device and control arms (Supplementary Table 7, Supplementary Table 8). Compared with GDMT alone, MitraClip consistently reduced the two-year rate of death or HFH in patients with HFrEF (HR 0.50, 95% CI: 0.38-0.68) and HFpEF (HR 0.58, 95% CI: 0.34-1.01); pint=0.63 (Figure 3, Supplementary Table 9). The MitraClip was also consistently effective both in patients with HFrEF and in those with HFpEF in reducing the individual endpoints of mortality and HFH, and improving MR severity, QOL, and 6MWD (Figure 3, Supplementary Table 9). Similar outcomes were observed in patients with LVEF ≤30% and >30% (Supplementary Table 10, Supplementary Figure 2). By spline analysis, the benefits of MitraClip in reducing death or HFH at two years were consistent across the range of LVEF enrolled (Figure 4); however, given small numbers at the margins the spline curves show uncertainty regarding the benefits of MitraClip in patients with the lowest (≤20%) and highest (>50%) LVEFs. The utility of the MitraClip in these groups requires further evaluation in future studies.
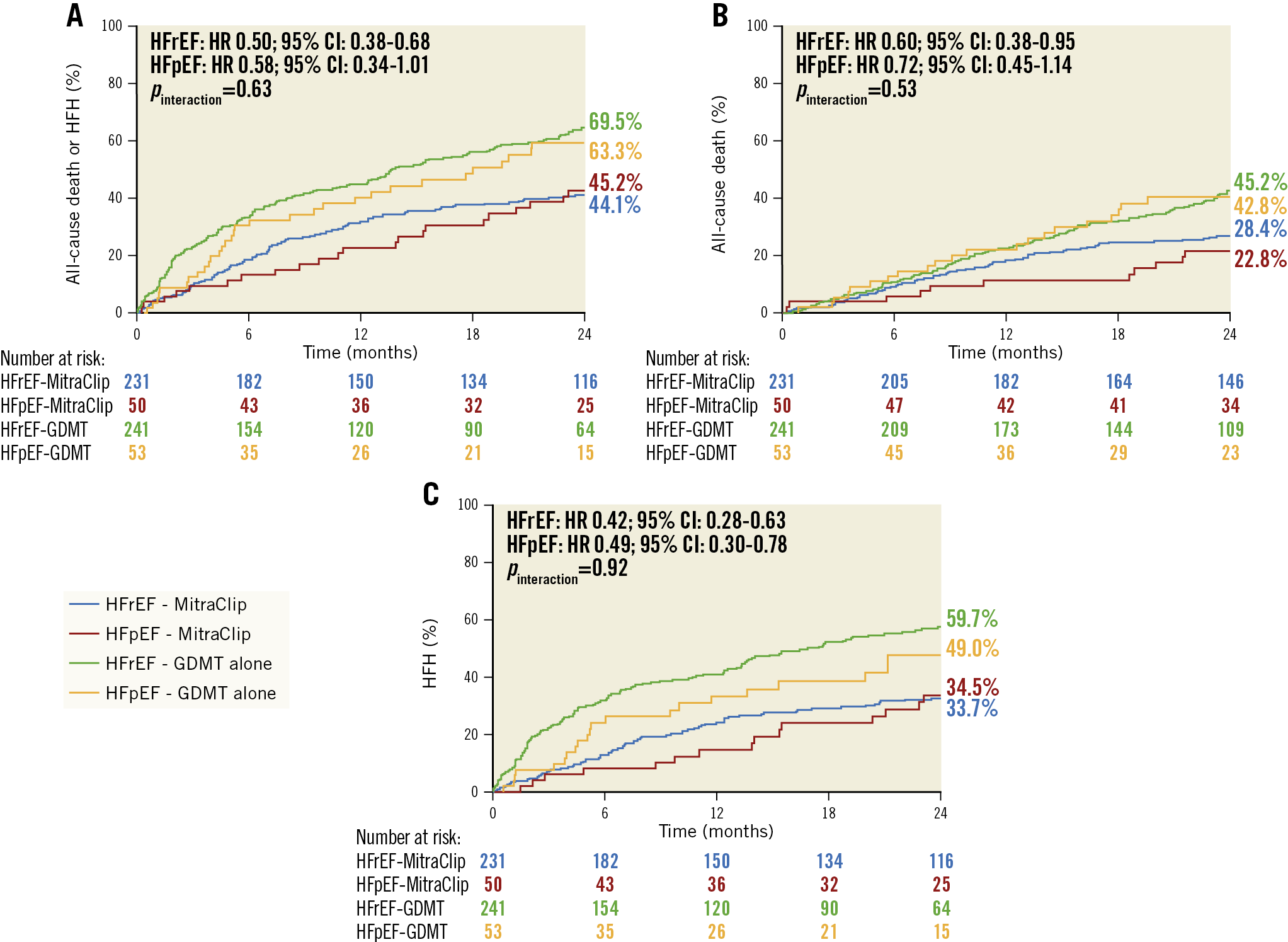
Figure 3. Kaplan-Meier time-to-first-event curves in patients randomised to MitraClip plus GDMT versus GDMT alone and with HFrEF versus HFpEF. A) Death or heart failure hospitalisation (HFH). B) Death. C) HFH. CI: confidence interval; GDMT: guideline-directed medical therapy; HFpEF: heart failure with preserved ejection fraction; HFrEF: heart failure with reduced ejection fraction; HR: hazard ratio
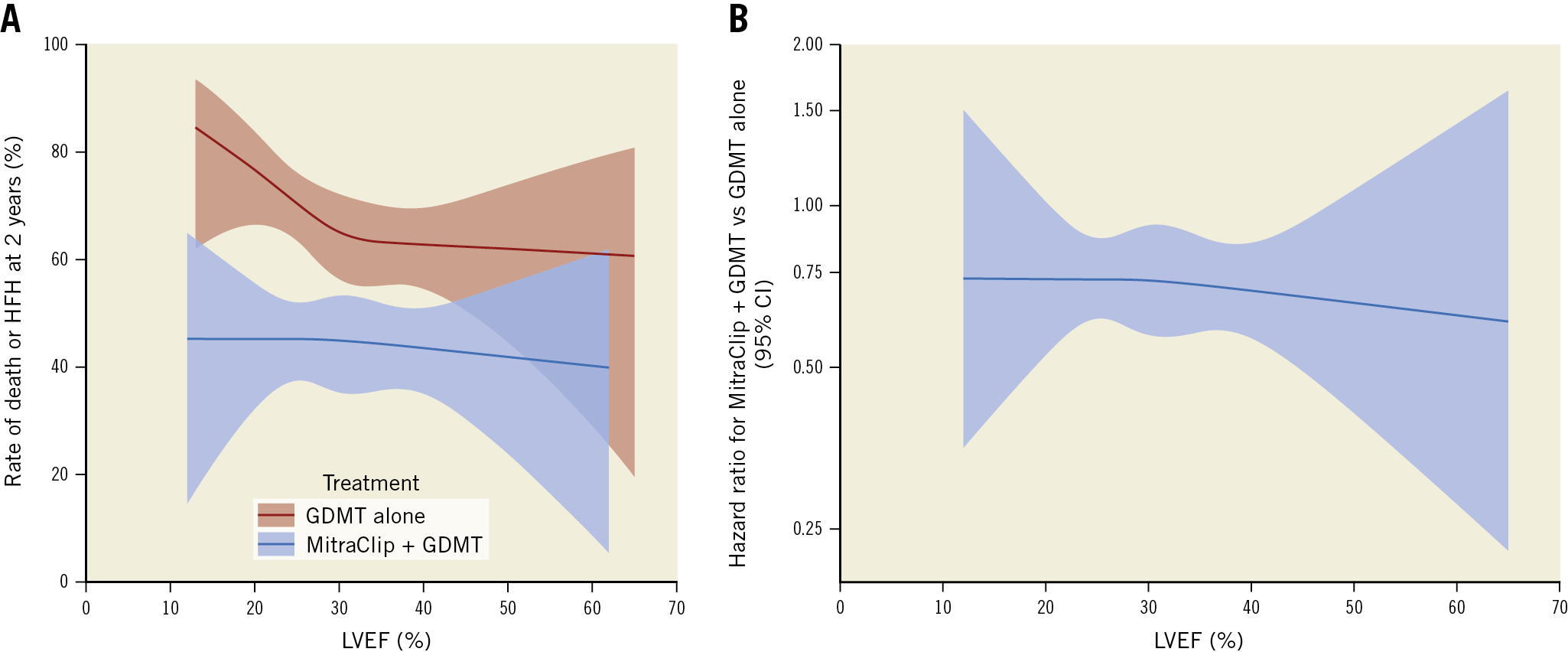
Figure 4. Spline analysis. A) Rate of death or heart failure hospitalisation (HFH) at two years according to baseline left ventricular ejection fraction (LVEF) in patients randomised to MitraClip plus guideline-directed medical therapy (GDMT) and GDMT alone. B) Hazard ratio for death or HFH at two years in patients treated with MitraClip plus GDMT versus GDMT alone according to baseline LVEF. CI: confidence interval
Discussion
SMR is in most cases a disease of the LV myocardium6. Patients with LV dysfunction who develop SMR of any magnitude of severity have a worse prognosis than those without MR16,17. MR reduces the resistance to LV ejection (afterload); as such, the LVEF in patients with severe MR underestimates the degree of LV systolic dysfunction18. SMR is a strong predictor of death even in patients with less severe HF19.
Whereas guidelines recommend early surgical intervention for severe primary MR6,8,20, the optimal timing of intervention for severe SMR is less clear21; surgical treatment for SMR has not been shown to improve prognosis22. Until recently, patients with HF and SMR were treated principally with medications targeted to the underlying LV myocardial dysfunction5,23. Recently, two multicentre randomised trials assessed the role of TMVr with the MitraClip in patients with HF and SMR. The MITRA-FR trial randomised patients with SMR with LVEF of 15% to 40% to GDMT plus TMVr versus GDMT alone1. At one-year follow-up, there was no significant difference between the two treatments for all-cause death (HR 1.11, 95% CI: 0.69-1.77) or HFH (HR 1.13, 95% CI: 0.81-1.56). The COAPT trial randomised patients with SMR with LVEF of 20% to 50% to GDMT plus TMVr versus GDMT alone3. At two-year follow-up, all HFHs were markedly lower in the MitraClip group compared with control (HR 0.53, 95% CI: 0.40-0.70; p<0.001). Death occurred in 29.1% of patients treated with TMVr compared with 46.1% treated with GDMT alone (HR 0.62, 95% CI: 0.46-0.82, p<0.001). While numerous differences between these trials may underlie their discordant findings, one common view has centred around the severity of MR relative to the degree of LV dysfunction. Patients in COAPT had more severe SMR compared with MITRA-FR (mean effective regurgitant orifice area 0.41 cm2 versus 0.31 cm2), yet smaller LV end-diastolic volumes4,5,6,13,21, signifying a greater proportion of patients with “disproportionately” severe MR in COAPT1,2.
Whereas LV dilatation was less pronounced in COAPT compared with MITRA-FR, the mean population LVEF was similar in the two studies (31.3% and 33.1%, respectively). In COAPT the benefits of MitraClip in terms of MR reduction, functional improvement, and freedom from death and HFH were consistent in those patients with a markedly depressed LVEF (≤30%) and less severely depressed LVEF (>30%) (mean LVEF in the two groups 23.9% and 39.0%, respectively). Moreover, nearly one in five COAPT patients had HFpEF (LVEF >40%), although most fell within the span of so-called HF with mid-range EF (HFmrEF), consistent with mildly reduced LV systolic function24. Data from the Acute Decompensated Heart Failure Syndromes (ATTEND) registry showed that even mild MR is associated with an increased risk of adverse outcomes in patients with HFpEF, while moderate to severe MR is associated with adverse outcomes in HFrEF25. The MitraClip consistently improved outcomes in this subgroup as well, which perhaps is not surprising given that HFmrEF patients have often responded to pharmacotherapies concordantly with HFrEF rather than HFpEF patients. In addition, given the afterload-reducing effect of severe MR, the severity of underlying LV systolic dysfunction in patients in COAPT with baseline LVEF of 40%-50% more likely resembles that of patients with LVEF <40% without MR. Thus, while the present study is reassuring that symptomatic patients with HF, LVEF ≤50%, and 3+ or 4+ MR will have an improved prognosis after MitraClip compared with GDMT alone, further studies are required to examine the outcomes of TMVr in a true HFpEF population, those with LVEF >50% (or >60%). In patients with truly normal LV function, the mechanism of SMR may more frequently be left atrial dysfunction with primary mitral annular dilatation often due to atrial fibrillation (so-called “atrial” functional MR)25,26 rather than classic SMR due to LV dilatation with mitral leaflet tethering. Atrial functional MR as a result of atrial fibrillation or HFpEF-induced left atrial remodelling and subsequent annular dilatation, when present, is associated with a worse prognosis26. As patients with pure atrial MR were excluded from COAPT, whether such cases respond equally well to the MitraClip is unknown (although we cannot exclude the possibility that atrial SMR was the predominant mechanism in a few patients with a high baseline LVEF).
Limitations
The principal limitations of the present study apply to the COAPT trial in general. Namely, the present results apply to patients who remained symptomatic despite maximally tolerated GDMT, in whom truly severe MR was present according to American Society of Echocardiography criteria27 but without severe LV dilatation or marked pulmonary hypertension or severe right ventricular failure. In addition, echocardiography may be inaccurate in measuring LVEF because of foreshortening, poor endocardial border detection, and geometric assumptions that must be applied27. The challenges in echocardiographic LVEF measurements are evidenced in the variations in LVEF measured by the sites and the echocardiographic core laboratory; by core laboratory assessment, 45 patients were enrolled with an LVEF <20% or >50%, outside the protocol limits. Cardiac magnetic resonance imaging, which may be a superior gold standard to measure LV volumes and LVEF, was not performed in the present study28. Finally, several of the subgroups evaluated in this study were small, and subgroup analysis is inherently underpowered. Our findings should therefore be considered hypothesis generating.
Conclusions
Among patients with HF and moderate-to-severe (3+) and severe (4+) SMR due to LV dysfunction, the MitraClip was consistently effective in improving prognosis and health status across the range of LVEF enrolled in the COAPT trial (intended LVEF 20%-50%, actual LVEF 12%-65%).
|
Impact on daily practice The findings of this COAPT substudy provide critical guidance for the treatment of a range of heart failure patients with symptomatic significant secondary mitral regurgitation. MitraClip on top of guideline-directed medical therapy (GDMT) consistently and effectively reduces the mortality and heart failure hospitalisation and improves the healthcare status of patients with severe and moderate left ventricular dysfunction compared to GDMT alone. |
Funding
The COAPT trial was sponsored by Abbott (Santa Clara, CA, USA).
Conflict of interest statement
F.M. Asch reports institutional research contracts for work as core lab director, with Edwards, Medtronic, BSC, LivaNova, Neovasc, Ancora, and MVRx. N.J. Weissman is associate director of an academic echocardiography core laboratory (MedStar Health Research Institute) with institutional contracts with Abbott, Neovasc, Ancora, Mitralign, Medtronic, Boston Scientific, Edwards Lifesciences, Biotronik, and LivaNova. S. Kar reports consulting fees/advisory board - Boston Scientific, consulting fees/stock equity - Valcare, consulting fees - W.L. Gore and Medtronic. D.S. Lim reports research grant support from Abbott, Edwards, Medtronic and Gore, being a consultant to Abbott, Edwards, Keystone Heart, Pipeline, Siemens, Valgen, and Venus, being on the advisory board of Ancora and Venus, and having equity in 510 Kardiac and Venus. B.K. Whisenant reports being a consultant to Edwards Lifesciences, Boston Scientific, Gore, and Neochord. P.A. Grayburn reports consulting fees from Abbott Vascular, Edwards Lifesciences, W.L. Gore, Medtronic and 4C Medical, and grant support from Abbott Vascular, Boston Scientific, Cardiovalve, Edwards Lifesciences, W.L. Gore, Medtronic, and Neochord. M.J. Rinaldi reports being on the advisory board of Abbott, Boston Scientific, Cordis and 4C Medical, teaching courses for Abbott and Edwards, consulting for Abbott, Boston, Edwards and Cordis, and research support/grant from Boston Scientific. S.K. Sharma reports being on the speakers bureau of Abbott Vascular, Boston Scientific, and Cardiovascular Systems, Inc. S.R. Kapadia reports stock options in NaviGate Cardiac Structures, Inc. V. Rajagopal reports being a consultant to Abbott Vascular, being on the steering committee of TRILUMINATE (Abbott), being on the screening committee of Intrepid (Medtronic), and being founder/CEO of Opus Medical Therapies. I.J. Sarembock reports being on the advisory board of and consultant to Boston Scientific. G.H.L. Tang reports personal fees from Abbott Structural Heart, Medtronic, and W.L. Gore & Associates. J.A. Lindenfeld reports grants from and being a consultant to AstraZeneca, grants from Sensible Medical and VoluMetrix, and being a consultant to Abbott, Boehringer Ingelheim, CVRx, Edwards Lifesciences, Impulse Dynamics, and V-Wave. W.T. Abraham reports consulting fees from Boehringer Ingelheim, Respicardia, Sensible Medical, CVRx, and Impulse Dynamics, and salary support from V-Wave Medical, all for work done in the field of heart failure. M.J. Mack reports grants from Abbott. G.W. Stone reports speaker or other honoraria from Cook, Terumo, Qool Therapeutics and Orchestra Biomed, being a consultant to Valfix, TherOx, Vascular Dynamics, Robocath, HeartFlow, Gore, Ablative Solutions, Miracor, Neovasc, V-Wave, Abiomed, Ancora, MAIA Pharmaceuticals, Vectorious, Reva, Matrizyme and Cardiomech, and equity/options from Ancora, Qool Therapeutics, Cagent, Applied Therapeutics, Biostar family of funds, SpectraWave, Orchestra Biomed, Aria, Cardiac Success, MedFocus family of funds, and Valfix. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

