Abstract
Background: A risk score was recently derived from the Cardiovascular Outcomes Assessment of the MitraClip Percutaneous Therapy for Heart Failure Patients with Functional Mitral Regurgitation (COAPT) Trial. However, external validation of this score is still lacking.
Aims: We aimed to validate the COAPT risk score in a large multicentre population undergoing mitral transcatheter edge-to-edge repair (M-TEER) for secondary mitral regurgitation (SMR).
Methods: The Italian Society of Interventional Cardiology (GIse) Registry of Transcatheter Treatment of Mitral Valve RegurgitaTiOn (GIOTTO) population was stratified according to COAPT score quartiles. The performance of the COAPT score for 2-year all-cause death or heart failure (HF) hospitalisation was evaluated in the overall population and in patients with or without a COAPT-like profile.
Results: Among the 1,659 patients included in the GIOTTO registry, 934 had SMR and complete data for a COAPT risk score calculation. The incidence of 2-year all-cause death or HF hospitalisation progressively increased through the COAPT score quartiles in the overall population (26.4% vs 44.5% vs 49.4% vs 59.7%; log-rank p<0.001) and COAPT-like patients (24.7% vs 32.4% vs 52.3% vs. 53.4%; log-rank p=0.004), but not in those with a non-COAPT-like profile. The COAPT risk score had poor discrimination and good calibration in the overall population, moderate discrimination and good calibration in COAPT-like patients and very poor discrimination and poor calibration in non-COAPT-like patients.
Conclusions: The COAPT risk score has a poor performance in the prognostic stratification of real-world patients undergoing M-TEER. However, after application to patients with a COAPT-like profile, moderate discrimination and good calibration were observed.
Introduction
Mitral transcatheter edge-to-edge repair (M-TEER) is a valuable therapeutic option for patients with secondary mitral regurgitation (SMR) who meet specific criteria derived from the Cardiovascular Outcomes Assessment of the MitraClip Percutaneous Therapy for Heart Failure Patients with Functional Mitral Regurgitation (COAPT) Trial12. COAPT was the first randomised controlled trial showing a prognostic benefit of M-TEER on top of guideline-directed medical therapy (GDMT) in patients with SMR and chronic heart failure (HF)3. On the other hand, the Multicenter Study of Percutaneous Mitral Valve Repair MitraClip Device in Patients With Severe Secondary Mitral Regurgitation (MITRA-FR) trial reported similar outcomes in patients with SMR receiving M-TEER and GDMT or GDMT alone4. Notably, in a real-world setting, there is a fair degree of overlap between the COAPT and MITRA-FR profiles5 so although selection for M-TEER is crucial for outcome purposes, identifying which patients will have a prognostic benefit from the procedure may be hard. Many efforts have been made to identify prognostic variables and to develop specific tools to predict clinical events after M-TEER6789.
A simple new risk model, derived from the COAPT population, was recently proposed to predict all-cause mortality or HF hospitalisation in patients with SMR and HF treated with either M-TEER and GDMT or GDMT alone10. The COAPT risk score has good performance in its derivation SMR cohort, but it has not yet been validated in external populations.
The Italian Society of Interventional Cardiology (GIse) Registry Of Transcatheter Treatment of Mitral Valve regurgitaTiOn (GIOTTO) is a large multicentre registry including patients undergoing M-TEER.
The aim of this study was to provide an external validation of the COAPT risk model in the SMR cohort of the GIOTTO registry.
Methods
Study population
The design and main results of the GIOTTO registry have been previously published1112. Briefly, GIOTTO is a multicentre, prospective, observational registry that included all M-TEER procedures performed at 18 participating sites from 2016 to 2021. The registry reflects the real-world experience of M-TEER with the MitraClip (Abbott) device (including the latest generations of the MitraClip, the XTR and NTR, and excluding the MitraClip G4) in both primary mitral regurgitation (PMR) and SMR, without strict selection criteria. For the purposes of the present analysis, from the original cohort, we selected those with SMR and with complete data for COAPT risk score calculation.
The COAPT risk score was recently presented as a new tool for predicting 2-year all-cause death or HF hospitalisation in patients with SMR and HF after M-TEER and GDMT (device group) or GDMT alone (control group)10. The score includes 4 clinical variables: i) chronic kidney disease (CKD) stage III (+1 point) or stage IV or greater (+3 points); ii) New York Heart Association (NYHA) Functional Class III or IVa (+1 point); iii) chronic obstructive pulmonary disease (COPD; +1 point); and iv) history of atrial fibrillation or flutter (+1 point); as well as 4 echocardiographic parameters: i) right ventricular systolic pressure (RVSP) >45 mmHg (+3 points); ii) left ventricle ejection fraction (LVEF) 25-35% (+1 point), LVEF <25% (+2 points); iii) left ventricular end-systolic diameter (LVESD) >5.5 cm (+2 points); and iv) tricuspid regurgitation (TR) >1+ (+2 points), in addition to MitraClip therapy (‒3 points). Thus, the score ranges between ‒3 (low risk) and +15 points (high risk). The COAPT risk score was calculated and assigned to each patient.
The population was stratified according to quartiles of COAPT risk score in the following groups: ‒3 to 1 points; 2 to 3 points; 4 to 5 points; and 6 to 12 points.
The impact of the COAPT risk score was evaluated in the overall SMR population, and after stratification, according to the presence of a COAPT-like profile as previously defined13.
Data collection and study outcomes
A web-based electronic case report form, periodically cross-checked for accuracy, was used for data collection. Subsequent follow-up data were obtained by outpatient clinical visits and/or telephone calls scheduled at 30 days, 1 year, and yearly thereafter.
Clinical outcomes were defined according to the Mitral Valve Academic Research Consortium (MVARC) criteria14. Procedural outcomes included acute technical success (defined as successful access, delivery and retrieval of the device delivery system; successful deployment of the device without procedural mortality or urgent surgery), 30-day device success (defined as optimal residual mitral regurgitation [rMR 1+] or an acceptable [rMR 2+] reduction in MR at 30 days after M-TEER), and 30-day procedural success (defined as a composite of device success and absence of major cardiovascular adverse events).
Procedural complications, mortality and hospitalisation were also reported according to MVARC definitions. The cause of hospitalisation, including HF, was site-reported.
In order to faithfully validate the COAPT risk score in our population, we considered the primary outcome to be the composite of 2-year all-cause death and HF hospitalisation after M-TEER.
The study complied with the Declaration of Helsinki and was approved by all the local ethical committees. All patients included in the study signed a written informed consent, after receiving an oral and written explanation of the risks and benefits concerning the procedure.
Statistical analysis
Categorical and dichotomous covariates are presented as counts and percentages and were compared by Pearson’s chi-square or Fisher’s exact tests with 2 degrees of freedom, as appropriate. Continuous covariates are presented as median and interquartile range (25th-75th IQR) and were compared using one-way analysis of variance (ANOVA) or a T-test, as appropriate.
A time-to-first-event analysis using the Kaplan-Meier method was performed to assess the 2-year cumulative incidence of all-cause death or HF hospitalisation in the population stratified by the COAPT risk score quartiles, and comparisons were made by means of the log-rank test.
A Cox regression analysis was performed to calculate the hazard ratio (HR) and corresponding 95% confidence interval (CI) of the primary outcome for the COAPT score as a dichotomic variable (4th, 3rd, 2nd quartiles vs 1st) and as a continuous variable for the COAPT risk score items.
The performance of the COAPT score was evaluated in terms of discrimination and calibration.
Discrimination was measured using the area under the curve (AUC) of the receiver operating characteristic (ROC), which ranged from 0.50 (no discrimination) to 1.0 (perfect discrimination). Model calibration was assessed using the Hosmer-Lemeshow (HL) goodness-of-fit test with a p-value of>0.05 indicating good calibration.
For all analyses, the primary outcome was evaluated in the overall SMR population and in patients both fulfilling and not fulfilling the COAPT-like profile. A two-sided p<0.05 was considered significant. Data were analysed by using the SPSS statistics software (version 21, IBM).
Results
Baseline characteristics
Among the 1,659 patients included in the GIOTTO registry, 934 had SMR and complete data for the COAPT risk score calculation and were included in this analysis (Figure 1). Baseline characteristics of the overall population, stratified by COAPT-like profile are reported in Supplementary Table 1. The COAPT risk score has a Gaussian distribution (Supplementary Figure 1). The median COAPT score was 4 (IQR 2-6) in the overall population, 1 (IQR 3-6) in patients fulfilling a COAPT-like profile and 3 (IQR 5-7) in those not fulfilling a COAPT-like profile. Distribution of the COAPT score items in the population stratified by COAPT-like profile is reported in Figure 2. A history of atrial fibrillation, LVEF <25%, RVSP >45 mmHg and TR >1+ were more frequently observed in patients with a non-COAPT-like profile as compared to those with a COAPT-like profile.
Baseline demographic and clinical characteristics of the overall population stratified by quartiles of the COAPT risk score are reported in Table 1. As expected, the prevalence of variables included in the COAPT risk model increased with the increasing COAPT score quartiles. A similar trend was observed for age, the European System for Cardiac Operative Risk Evaluation (EuroSCORE II), the Society of Thoracic Surgeons (STS) score, levels of N-terminal pro-B-type natriuretic peptide (NT-proBNP), prevalence of hypertension, peripheral artery disease (PAD) and severity of MR. Moreover, body mass index (BMI) and haemoglobin were lower in the 4th quartile of the COAPT score compared with the others (Table 1).
Similar differences were observed in the COAPT-like and non-COAPT-like populations stratified by quartiles of COAPT risk score (Supplementary Table 2, Supplementary Table 3).
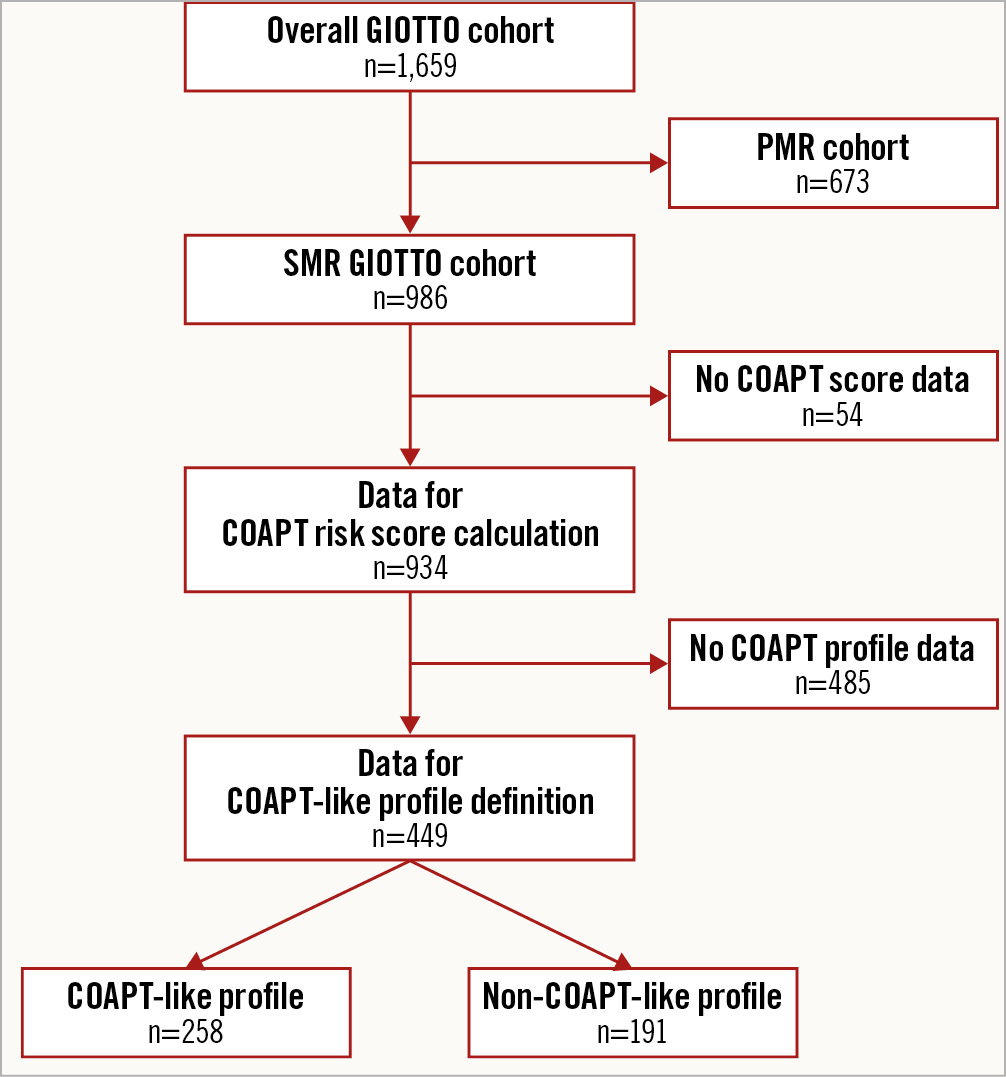
Figure 1. Study flowchart. Patients excluded and included in the present analysis are reported. COAPT: Cardiovascular Outcomes Assessment of the MitraClip Percutaneous Therapy for Heart Failure Patients With Functional Mitral Regurgitation; GIOTTO: Italian Society of Interventional Cardiology (GIse) Registry of Transcatheter Treatment of Mitral Valve RegurgitaTiOn; PMR: primary mitral regurgitation; SMR: secondary mitral regurgitation
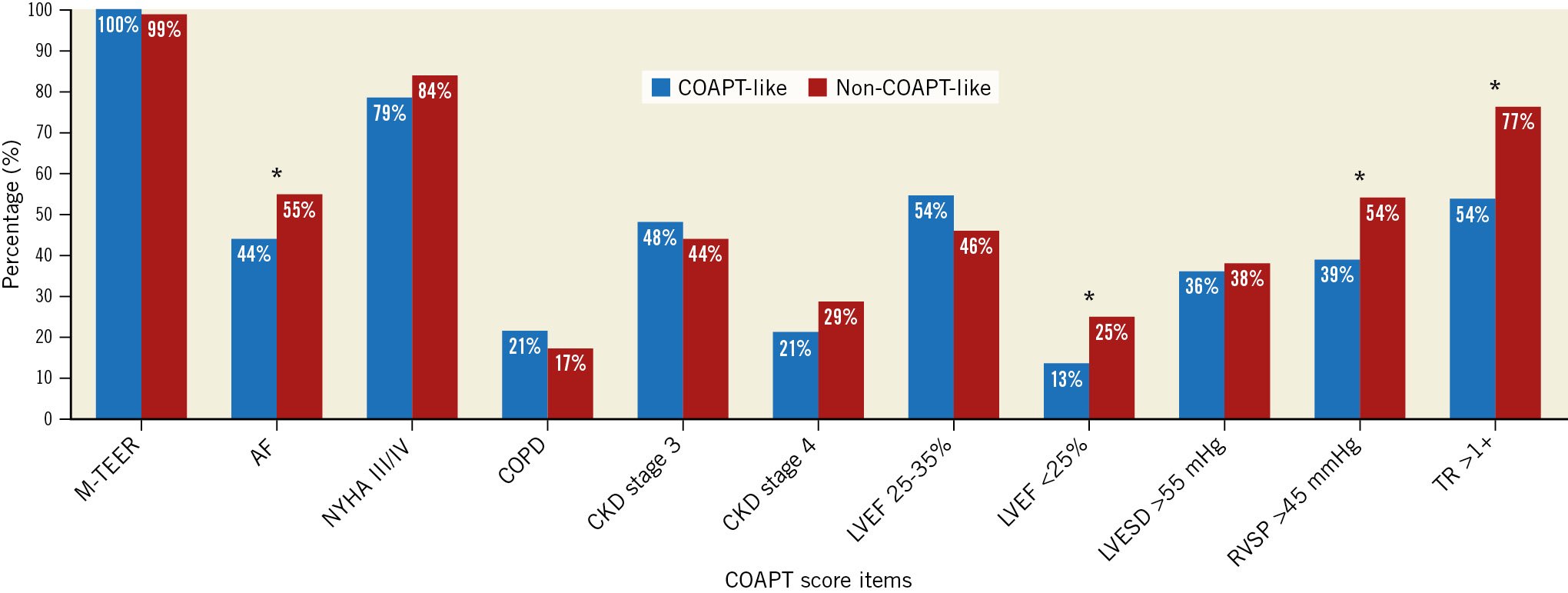
Figure 2. Proportion of patients with and without a COAPT-like profile presenting different items included in the COAPT risk score. *indicates p<0.05 in the comparison between groups. AF: atrial fibrillation; CKD: chronic kidney disease; COAPT: Cardiovascular Outcomes Assessment of the MitraClip Percutaneous Therapy for Heart Failure Patients With Functional Mitral Regurgitation; COPD: chronic obstructive pulmonary disease; LVEF: left ventricular ejection fraction; LVESD: left ventricular end-systolic diameter; M-TEER: mitral transcatheter edge-to-edge repair; NYHA: New York Heart Association; RVSP: right ventricular systolic pressure; TR: tricuspid regurgitation
Table 1. Baseline characteristics according to COAPT score quartiles.
| COAPT score–3 to 1 (n=229) | COAPT score2 to 3 (n=207) | COAPT score4 to 5 (n=243) | COAPT score6 to 12 (n=255) | p-value | |
|---|---|---|---|---|---|
| Age, years | 71 [70-72] | 74 [73-75] | 73 [72-74] | 74 [73-75] | 0.001 |
| Male sex | 151 (66) | 143 (69) | 177 (73) | 189 (74) | 0.192 |
| BMI, kg/m² | 25.8 [25.2-26.3] | 25.6 [25.1-26.2] | 25.5 [24.9-25.9] | 24.9 [24.4-25.4] | 0.070 |
| EuroSCORE II, % | 5.9 [5.3-6.5] | 7.3 [6.4-8.2] | 7.1 [6.4-7.8] | 10.5 [9.5-11.6] | 0.001 |
| STS risk score, % | 3.6 [2.8-4.3] | 4.5 [3.6-5.4] | 5.1 [4.1-6.0] | 6.5 [5.4-7.6] | 0.001 |
| NYHA Class III/IV | 157 (69) | 173 (84) | 216 (89) | 233 (91) | 0.001 |
| Comorbidities, laboratory data and medical therapies | |||||
| Hypertension | 162 (71) | 154 (74) | 193 (79) | 175 (69) | 0.039 |
| Diabetes mellitus | 84 (37) | 70 (34) | 80 (33) | 85 (33) | 0.825 |
| Prior HF | 145 (63) | 143 (69) | 164 (67) | 190 (74) | 0.064 |
| COPD | 19 (8) | 27 (13) | 42 (17) | 49 (19) | 0.004 |
| Prior cerebrovascular disease | 13 (6) | 21 (10) | 20 (8) | 23 (9) | 0.364 |
| AF | 72 (31) | 102 (49) | 138 (57) | 153 (60) | 0.001 |
| CAD | 129 (56) | 109 (53) | 119 (49) | 153 (60) | 0.082 |
| Prior cardiac surgery | 73 (32) | 61 (29) | 51 (21) | 66 (26) | 0.087 |
| PAD | 22 (10) | 15 (7) | 20 (8) | 28 (11) | 0.006 |
| GFR <30 mL/min | 12 (5) | 34 (16) | 48 (20) | 115 (45) | 0.001 |
| 30 mL/min ≤ GFR ≤ 60mL/min | 113 (49) | 118 (57) | 126 (52) | 112 (44) | 0.001 |
| GFR >60 mL/min | 104 (45) | 55 (27) | 69 (28) | 28 (11) | 0.001 |
| Haemoglobin, g/dL | 12.6 [12.3-12.8] | 12.4 [12.2-12.7] | 12.3 [12.0-12.6] | 12.0 [11.8-12.3] | 0.017 |
| Creatinine, mg/dL | 1.25 [1.18-1.31] | 1.49 [1.38-1.60] | 1.57 [1.45-1.68] | 1.97 [1.84-2.11] | 0.001 |
| NT-proBNP, ng/L | 1,258 [808-1,707] | 1,463 [933-1,993] | 2,520 [1,785-3,254] | 3,496 [2,714-4,278] | 0.001 |
| CRT | 66 (29) | 94 (45) | 99 (41) | 141 (55) | 0.001 |
| Beta blocker | 190 (83) | 172 (83.1) | 207 (85.2) | 212 (83.1) | 0.899 |
| ACEi/ARB/ARNI | 89 (38.9) | 71 (34.3) | 87 (35.8) | 83 (32.5) | 0.525 |
| MRA | 125 (54.6) | 116 (56) | 139 (57.2) | 138 (54.1) | 0.900 |
| Furosemide | 207 (90.4) | 194 (93.7) | 232 (95.5) | 239 (93.7) | 0.164 |
| Echocardiographic data | |||||
| LAD, mm | 56 [46-65] | 53 [46-59] | 54 [48-60] | 54 [48-60] | 0.945 |
| LVEDD, mm | 60 [58-61] | 62 [61-64] | 63 [61-64] | 66 [65-67] | 0.001 |
| LVESD, mm | 46 [45-48] | 50 [48-51] | 48 [47-50] | 55 [53-56] | 0.001 |
| LVEDV, mL | 156 [149-164] | 175 [165-185] | 176 [167-184] | 193 [185-201] | 0.001 |
| LVEDVi, mL/m² | 86 [82-90] | 97 [91-102] | 96 [91-100] | 108 [103-112] | 0.001 |
| LVESV, mL | 98 [92-104] | 116 [108-125] | 114 [107-121] | 137 [130-144] | 0.001 |
| LVESVi, mL/m² | 54 [51-57] | 64 [60-69] | 62 [58-66] | 77 [73-80] | 0.001 |
| LVEF, % | 39 [37-40] | 34 [32-35] | 34 [33-36] | 29 [28-30] | 0.001 |
| MR 2+ | 5 (2.2) | 2 (1) | 3 (1.2) | 1 (0.4) | 0.330 |
| MR 3+ | 74 (32) | 61 (29) | 39 (16) | 42 (16) | 0.001 |
| MR 4+ | 150 (50) | 144 (69) | 201 (83) | 212 (83) | 0.001 |
| MVA planimetry, cm² | 4.9 [4.7-5.1] | 4.9 [4.8-5.2] | 5.2 [4.9-5.4] | 5.2 [4.9-5.4] | 0.286 |
| Severe TR | 12 (5) | 18 (9) | 34 (14) | 52 (20) | 0.001 |
| TAPSE, mm | 19 [19-20] | 19 [18-19] | 18 [17-19] | 18 [16-19] | 0.041 |
| sPAP, mmHg | 37 [36-38] | 42 [41-44] | 52 [50-53] | 57 [55-58] | 0.001 |
| Values are expressed as n (%), or median [interquartile range]. ACEi/ARB/ARNI: angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers or angiotensin receptor/neprilysin inhibitors; AF: atrial fibrillation; BMI: body mass index; CAD: coronary artery disease; COAPT: Cardiovascular Outcomes Assessment of the MitraClip Percutaneous Therapy for Heart Failure Patients With Functional Mitral Regurgitation; COPD: chronic obstructive pulmonary disease; CRT: cardiac resynchronisation therapy; EuroSCORE II: European System for Cardiac Operative Risk Evaluation; GFR: glomerular filtration rate; HF: heart failure; LAD: left atrial diameter; LVEDD: left ventricular end-diastolic diameter; LVEDVi: left ventricular end-diastolic volume index; LVEF: left ventricular ejection fraction; LVESD: left ventricular end-systolic diameter; LVESVi: left ventricular end-systolic volume index; MR: mitral regurgitation; MRA: mineralocorticoid receptor antagonist; MVA: mitral valve area; NT-proBNP: N-terminal pro-B-type natriuretic peptide; NYHA: New York Heart Association; OMT: optimal medical therapy; PAD: peripheral artery disease; sPAP: systolic pulmonary artery pressure; STS: Society of Thoracic Surgeons; TAPSE: tricuspid annular plane systolic excursion; TR: tricuspid regurgitation | |||||
Acute outcomes
Acute outcomes are reported in Table 2. Residual MR 1+ was more frequent in the lowest COAPT score quartiles, whereas residual MR 2+ and 3/4+ were more frequent in the highest COAPT score quartiles. The rate of in-hospital acute kidney injury, the need for an intra-aortic balloon pump and length of the ward stay were higher in the 4th COAPT score quartile compared with the others.
Table 2. Procedural and in-hospital outcomes.
| COAPT score–3 to 1 (n=229) | COAPT score2 to 3 (n=207) | COAPT score4 to 5 (n=243) | COAPT score6 to 12 (n=255) | p-value | ||
|---|---|---|---|---|---|---|
| Device time, min | 60 [35-90] | 50 [30-90] | 58 [35-85] | 60 [35-90] | 0.537 | |
| Procedural time, min | 100 [140-190] | 90 [120-170] | 135 [90-180] | 145 [95-190] | 0.373 | |
| Clip(s) implanted | 1 | 93 (40.6) | 76 (36.7) | 84 (34.6) | 92 (36) | 0.573 |
| 2 | 117 (51.1) | 115 (55.6) | 133 (54.7) | 130 (51) | 0.662 | |
| 3 | 16 (7) | 16 (7.7) | 23 (9.5) | 27 (10.6) | 0.469 | |
| 4 | 1 (0.4) | 0 (0) | 1 (0.4) | 4 (1.6) | 0.163 | |
| Failed implantation | 2 (0.9) | 0 (0) | 2 (0.8) | 2 (0.8) | 0.629 | |
| Procedural outcomes | ||||||
| Technical success | 223 (97.4) | 201 (97.1) | 235 (96.7) | 249 (97.6) | 0.932 | |
| Device success | 206 (90) | 180 (87) | 212 (87.2) | 213 (83.5) | 0.219 | |
| Procedural success | 200 (87.3) | 177 (85.5) | 205 (84.4) | 207 (81.2) | 0.294 | |
| Intraprocedural death | 0 (0) | 0 (0) | 1 (0.4) | 1 (0.4) | 0.472 | |
| Clip detachment | 1 (0.4) | 2 (1) | 3 (1.2) | 2 (0.8) | 0.818 | |
| Residual MR 1+ | 171 (74.7) | 130 (62.8) | 148 (60.9) | 156 (61.2) | 0.001 | |
| Residual MR 2+ | 55 (24) | 70 (33.8) | 82 (33.7) | 90 (35.3) | ||
| Residual MR 3+/4+ | 3 (1.3) | 7 (3.4) | 13 (13.6) | 9 (3.5) | ||
| Mean gradient | 3 [2-4] | 3 [2.8-4.5] | 3 [2-4] | 3 [2-4] | 0.117 | |
| In-hospital outcomes | ||||||
| Vascular complications | 10 (4.4) | 9 (4.3) | 6 (2.5) | 6 (2.4) | 0.431 | |
| Major bleeding | 4 (1.7) | 4 (1.9) | 6 (2.5) | 9 (3.5) | 0.585 | |
| Cardiac tamponade | 0 (0) | 0 (0) | 1 (0.4) | 1 (0.4) | 0.624 | |
| Myocardial infarction | 0 (0) | 1 (0.5) | 0 (0) | 0 (0) | 0.319 | |
| AKI | 7 (3.1) | 6 (2.9) | 2 (0.8) | 17 (6.7) | 0.004 | |
| Malignant arrhythmia | 1 (0.4) | 1 (0.5) | 0 (0) | 1 (0.4) | 0.780 | |
| Stroke | 0 (0) | 2 (1) | 1 (0.4) | 1 (0.4) | 0.494 | |
| LVAD implantation | 0 (0) | 0 (0) | 0 (0) | 1 (0.4) | 0.446 | |
| IABP | 0 (0) | 0 (0) | 0 (0) | 4 (1.6) | 0.013 | |
| Need for urgent surgery | 1 (0.4) | 0 (0) | 1 (0.4) | 1 (0.4) | 0.834 | |
| ICCU length of stay, hours | 24 [0-43] | 24 [2-48] | 24 [0-48] | 24 [0-48] | 0.095 | |
| Ward length of stay, days | 3 [5-7] | 4 [3-7] | 5 [4-8] | 6 [4-10] | 0.001 | |
| Values are expressed as n (%), or median [interquartile range]. AKI: acute kidney injury; COAPT: Cardiovascular Outcomes Assessment of the MitraClip Percutaneous Therapy for Heart Failure Patients With Functional Mitral Regurgitation; IABP: intra-aortic balloon pump; ICCU: intensive coronary care unit; LVAD: left ventricular assistant device; MR: mitral regurgitation | ||||||
Two-year outcomes
Clinical follow-up data were available for 833 out of 934 patients (182/191 COAPT-like and 231/258 non-COAPT-like). The median follow-up was 370 (184-734) days with 538 patients (65%) reaching 2-year follow-up. The primary outcome occurred in 253 patients within the 2-year follow-up. The cumulative incidence of 2-year all-cause death or HF hospitalisation progressively increased through the COAPT score quartiles (26.4% vs 44.5% vs 49.4% vs 59.7%; log-rank p<0.001) (Figure 3). Similar results were observed in patients fulfilling a COAPT-like profile (24.7% vs 32.4% vs 52.3% vs 53.4%; log-rank p=0.004), whereas different trends were noted in patients who did not fulfil a COAPT-like profile (25.4% vs 59.3% vs 57.6% vs 49.1%; log-rank p=0.048) (Figure 3). The relative risk of the primary outcome increased with the increasing of the COAPT score in the overall population (Supplementary Figure 1, Table 3) and in the COAPT-like subgroup but not in the non-COAPT-like subgroup, even after adjustment for possible confounders (Table 3).
The association between single COAPT risk score items and the primary outcome was reported in Supplementary Table 4. Device success did not affect the impact of the COAPT risk score on the primary endpoint (p for interaction=0.858), but there was a trend towards a decreased risk of clinical events in patients with device success (HR 0.75, 95% CI: 0.55-1.01; p=0.061) regardless of the COAPT score.
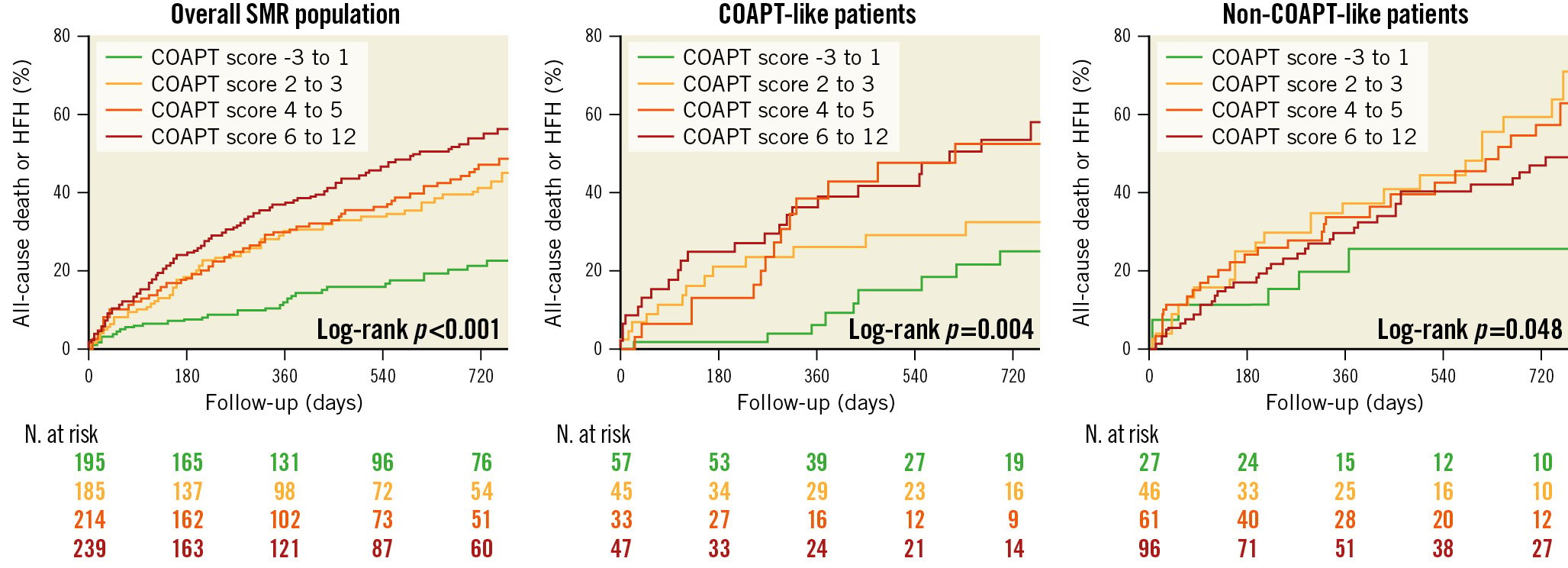
Figure 3. Cumulative incidence of 2-year all-cause mortality or HF hospitalisation in the overall population, in COAPT-like patients and in non-COAPT-like patients. COAPT: Cardiovascular Outcomes Assessment of the MitraClip Percutaneous Therapy for Heart Failure Patients With Functional Mitral Regurgitation; HFH: heart failure hospitalisation; SMR: secondary mitral regurgitation
Table 3. Association between COAPT risk score and relative risk of 2-year all-cause death or HF hospitalisation.
| HR | 95% CI | p-value | Adj HR* | 95% CI | p-value | |
|---|---|---|---|---|---|---|
| Overall population | 1.21 | 1.08-1.67 | <0.001 | 1.09 | 1.05-1.13 | <0.001 |
| COAPT-like profile | 1.15 | 1.06-1.27 | <0.001 | 1.14 | 1.06-1.23 | <0.001 |
| Non-COAPT-like profile | 0.99 | 0.93-1.06 | 0.769 | 0.97 | 0.90-1.04 | 0.425 |
| *Variables included in the model: age, gender, EuroSCORE II, mitral regurgitation grade. CI: confidence interval; COAPT: Cardiovascular Outcomes Assessment of the MitraClip Percutaneous Therapy for Heart Failure Patients With Functional Mitral Regurgitation; EuroSCORE II: European System for Cardiac Operative Risk Evaluation; HF: heart failure; Adj HR: adjusted hazard ratio | ||||||
Performance of the COAPT score
The COAPT risk score had poor discrimination power and good calibration in predicting 2-year all-cause death or HF hospitalisation in the overall population (AUC 0.62; HL p=0.658). Moderate discrimination and good calibration were noted in the COAPT-like subgroup (AUC 0.67; HL p=0.952). On the other hand, a very poor performance was observed in the non-COAPT-like subgroup (AUC 0.48; HL p=0.012) (Central illustration).
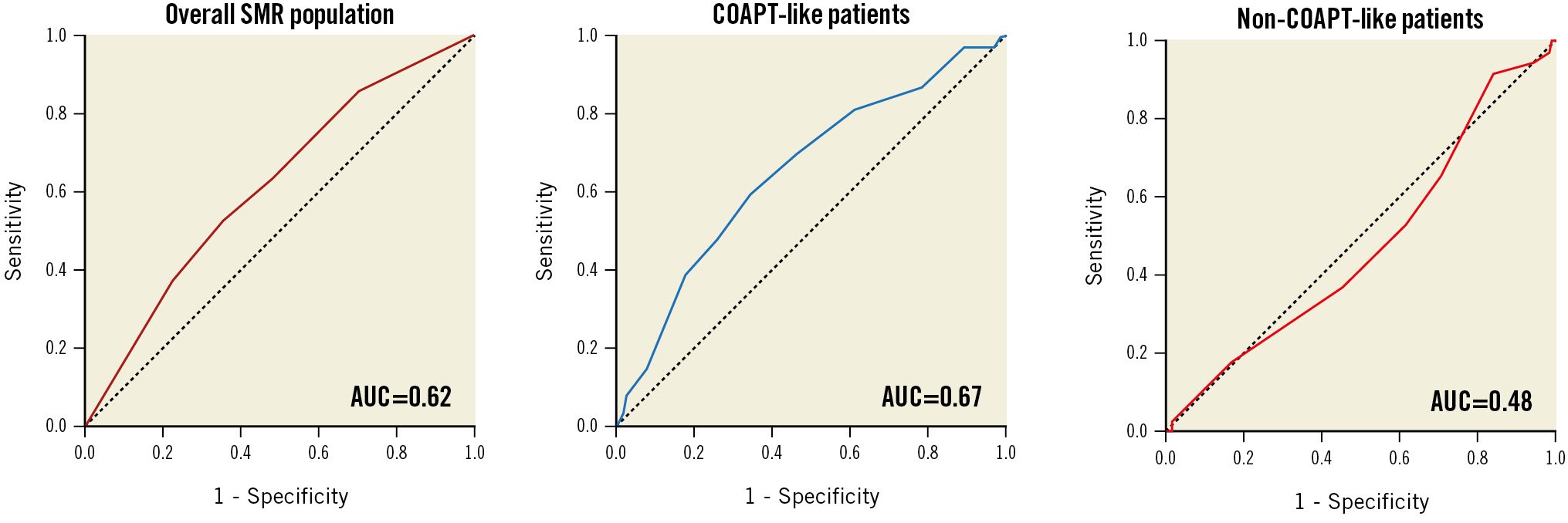
Central illustration. Receiver operating characteristic curves for the association between COAPT risk score and primary outcome in the overall population, in COAPT-like patients and in non-COAPT-like patients AUC: area under the curve; SMR: secondary mitral regurgitation
Discussion
The key finding of this study is that the recently proposed COAPT risk score has a poor performance in the prognostic stratification of real-world patients undergoing M-TEER. However, in patients with a COAPT-like profile, the COAPT score had moderate discrimination and good calibration in predicting 2-year all-cause death or HF hospitalisation.
Patient selection for M-TEER remains challenging in spite of many efforts to identify predictors of outcomes891516. Since surgical risk scores overpredict mortality in these patients17, some specific models have been developed to predict the risk of adverse events after M-TEER671018. Shah et al recently proposed a risk score derived from the COAPT population, including 614 patients with SMR treated with optimised HF therapies, enrolled at 78 centres in the United States and Canada and randomised to receive M-TEER or not. The endpoint used to build the model was the 2-year rate of death or HF hospitalisation. M-TEER was strongly associated with a reduced risk of events. Moreover, 4 clinical variables, 4 echocardiographic parameters, and the MitraClip treatment, were identified as independently associated with the endpoint of interest in the multivariate analysis. Thus, 9 variables were included in the risk score with a final score ranging from ‒3 to 1210.
All the included variables have already been reported as associated with outcome in M-TEER populations58131519202122232425262728. However, the COAPT risk model may represent a user-friendly tool to easily perform a comprehensive patient evaluation and selection in clinical practice29.
We validated the COAPT score in a large multicentre real-world population included in the GIOTTO registry. Overall, the COAPT and GIOTTO populations are extremely different, as the former included only SMR patients undergoing M-TEER or conservative care, whereas the latter included both SMR and PMR patients undergoing M-TEER. To partially overcome this issue, we included only SMR patients in this analysis, but several differences between the device arm of COAPT and the SMR group of GIOTTO had already been reported11. Briefly, GIOTTO patients had more advanced symptoms, severe SMR and were less likely to receive angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers or angiotensin receptor/neprilysin inhibitors compared to COAPT patients. Moreover, in COAPT but not in GIOTTO, patients with a non-ambulatory NYHA Class IV were excluded. and optimisation of HF medical therapies was centrally evaluated by a dedicated committee. In addition, while patients with severe TR were excluded from COAPT, 15% of SMR patients in GIOTTO had severe TR. Importantly, in our analysis, NYHA Class III-IV and TR >1+ were the most frequent items observed among clinical and echocardiographic items, respectively.
Aware of these disparities between the COAPT population and our validation cohort, we evaluated the COAPT score in patients with and without a COAPT-like profile. As expected, the performance of the score was significantly better in the former as compared to the latter group. However, the performance of the COAPT score observed in the derivation cohort (AUC 0.74; HL p=0.97) was still better than that observed in our COAPT-like population (AUC 0.67; HL p=0.95). This could be explained by the fact that clinical events in GIOTTO are not centrally adjudicated and HF hospitalisations are probably underestimated because of underreporting. Indeed, while the mortality rate was similar between the COAPT and SMR GIOTTO populations, the HF hospitalisation rate was lower in SMR GIOTTO versus COAPT, and the cumulative incidence of the composite endpoint was lower through the COAPT risk score quartiles in GIOTTO versus COAPT. Moreover, the follow-up length was shorter in our population as compared to COAPT, with only 65% reaching 2-year follow-up. Finally, only half of the patients with SMR and data for a COAPT risk score calculation also had data for a COAPT-like profile definition (Figure 1), mainly because of a high rate of missing values for right ventricular function. Therefore, the low number of patients included in the analysis as well as the lower number of events in GIOTTO versus COAPT, due to the underreported HF hospitalisations and the shorter follow-up, may have affected the poorer performance of the COAPT score in predicting clinical events in our COAPT-like population compared with the derivation cohort.
Nevertheless, we can conclude that the COAPT risk score might reasonably be used for prognostic stratification of COAPT-like patients with SMR undergoing M-TEER. On the other hand, in SMR patients not fulfilling COAPT criteria, other recently proposed tools30 should be used, since the performance of the COAPT risk score in this subgroup of patients is very poor.
Limitations
Several limitations of this study must be acknowledged. This is an observational registry whose variables were site-reported, and both clinical outcomes and echocardiographic data were not adjudicated by a central committee and a core laboratory, respectively. However, the echocardiographers involved in GIOTTO were all accredited by the Italian Society of Echocardiography (SIECVI), and all examinations were performed in accordance with its required standards. Moreover, guideline-directed optimal medical therapy was not established in all patients before M-TEER as in COAPT. In addition, complete 2-year follow-up data were not available for the overall population as in COAPT. Indeed, among patients with clinical follow-up data available (89%), only 65% reached 2-year follow-up. Finally, being site-reported and not centrally evaluated, HF hospitalisations may be underreported since the incidence is lower compared to the COAPT study. These limitations may explain the lower performance of the COAPT risk score in the GIOTTO population as compared to the derivation cohort.
Conclusions
In a large real-world SMR population undergoing M-TEER, the recently proposed COAPT risk score had a poor performance for the prediction of 2-year all-cause mortality or HF hospitalisation. However, moderate discrimination and good calibration were observed in the subgroup of patients fulfilling a COAPT-like profile, whereas a very poor performance was observed in non-COAPT-like patients.
Impact on daily practice
The COAPT risk score may be useful for the prognostic stratification of patients with a COAPT-like profile undergoing mitral transcatheter edge-to-edge repair. On the other hand, its performance is poor when applied to non-COAPT like patients.
Funding
The GIOTTO registry is sponsored by the Italian Society of Interventional Cardiology (GIse), which received grant support for the study from Abbott Vascular.
Conflict of interest statement
M. Adamo received consultation and/or speaker fees from Abbott Vascular, outside the submitted work. F. Bedogni received consultation and/or speaker fees from Abbott Vascular, outside the submitted work. P. Denti received consultation and/or speaker fees from Abbott Vascular, outside the submitted work. C. Grasso received consultation and/or speaker fees from Abbott Vascular, outside the submitted work. A. Giordano received consultation and/or speaker fees from Abbott Vascular, outside the submitted work. F. De Marco, A.S. Petronio and G. Tarantini received consultation and/or speaker fees from Abbott Vascular outside the submitted work. M. Metra received consultation and/or speaker fees from Abbott Vascular, outside the submitted work. C. Tamburino received consultation and/or speaker fees from Abbott Vascular, outside the submitted work. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

