Abstract
Aims: We report a case of emergency transcatheter heart valve implantation in a failing mitral bioprosthesis via a transseptal access complicated by the atrial migration of a prosthesis.
Methods and results: A 42-year-old woman was referred for stenotic failure of a mitral bioprosthesis. A transapical valve-in-valve implantation was initially planned. However, due to sudden haemodynamic deterioration, an emergency transseptal implantation via a femoral venous access was undertaken. Following cardiac arrest, the procedure was performed with extracorporeal membrane oxygenation (ECMO), and was complicated by the migration of a valve, which was left moving freely in the left atrium. A second valve was successfully implanted in the mitral bioprosthesis. Following initial clinical recovery, there was a sudden recurrence of heart failure due to entrapment of the migrated valve in the implanted valve in a “reverse position”, which was dislodged percutaneously in an emergency procedure. The valve later migrated into the left atrial appendage. Immediate outcome was uneventful, but the patient suddenly died six months later.
Conclusions: Transseptal transcatheter mitral valve-in-valve implantation is feasible, even in an emergency setting with ECMO. Valve migration in the left atrium may occur and lead to late entrapment in a “reverse position”, with significant haemodynamic consequences.
Introduction
Transcatheter valve-in-valve (VIV) implantation for failing bioprostheses has recently emerged as a suitable alternative to reoperation in high-risk patients1,2. Implantation of transcatheter heart valves (THV) in the mitral position is particularly challenging due to difficult access, and to date this procedure has been performed approximately one hundred times worldwide, mostly through a transapical approach3. This approach has been favoured due to initial discouraging experiences from transseptal and transatrial routes1. However, recent attempts at THV implantations in the mitral position through the transseptal approach have proved to be successful4,5.
Moreover, patients with valve dysfunction may occasionally present with cardiogenic shock. Emergency balloon valvuloplasty for severe aortic and mitral stenosis may be lifesaving in these situations6-8. More recently, THV implantation has also been reported in this particular setting9,10.
Case report
We describe the emergency implantation of a THV valve in a failing mitral bioprosthesis from a transseptal approach in a patient with cardiogenic shock.
A 42-year-old woman from Tahiti in French Polynesia was referred to our hospital for recurrent pulmonary oedema secondary to stenotic degeneration of a mitral bioprosthesis. She had undergone mitral valve replacement five times since the age of twelve following rheumatic heart disease. Although complete information on the reasons for the multiple interventions was not available from the patient’s records, at least two of the operations were due to mechanical valve thrombosis because of poor compliance with the anticoagulation treatment. The last operation, which had been performed ten years earlier with the implantation of a Carpentier-Edwards 31 mm bioprosthesis (Edwards Lifesciences, Irvine, CA, USA), was extremely difficult with a complicated postoperative course.
Transthoracic and transoesophageal echocardiography (TOE) showed a severe stenosis of the mitral bioprosthesis (mean gradient 23 mmHg, valve area 0.5 cm2), with an almost complete fusion of the leaflets (Figure 1A, Moving image 1). The left ventricular dimensions and ejection fraction were normal. However, there was severe dilatation and dysfunction of the right ventricle. The left atrium was severely dilated with a volume of 181 ml. Measurement of the inner diameter of the prosthesis was 25.5 mm on three-dimensional (3-D) TOE, 27.1×27.3 mm on computed tomography scanning, and 26.7×27.5 mm by fluoroscopy (Figure 1B-Figure 1D).
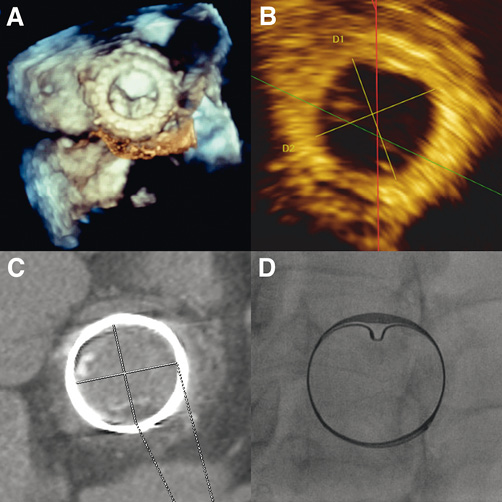
Figure 1. A) Left atrial 3-D TOE view of the stenotic mitral bioprosthesis. Measurement of the bioprosthesis annulus diameter by 3-D TOE (25.5 mm) (B), computed tomography scanner (26.7×27.5 mm) (C), and fluoroscopy (26.7×27.5 mm) (D).
The estimated risk of operative mortality of a new surgical mitral valve replacement was 11% and 3% according to the logistic EuroSCORE and Society of Thoracic Surgeons-Predicted Risk of Mortality (STS-PROM), respectively. Yet, due to the multiple operations she had previously undergone and the complicated postoperative course of the last surgery, the Heart Team considered that the patient was at high risk for a redo operation. This opinion was also shared by the team which had performed the last operation and had declined the patient before referral to our institution. It was therefore decided to perform a transapical mitral VIV implantation with a 29 mm balloon-expandable Edwards SAPIEN XT (Edwards Lifesciences) transcatheter heart valve (THV).
However, during the screening phase, the patient presented several episodes of rapid atrial fibrillation, which became complicated with haemodynamic instability and shock requiring orotracheal intubation and high-dose inotropic support. The patient’s condition further deteriorated within a few hours, with multiple organ failure (including hepatic failure) and disseminated intravascular coagulation (DIC). At this point, the risk of a rescue surgical intervention was extreme (logistic EuroSCORE 55%, STS-PROM 71%). Moreover, a transapical intervention was not considered feasible anymore due to the haemorrhagic risk. Therefore, it was decided to attempt an emergency mitral VIV implantation via a transseptal approach with a 26 mm SAPIEN XT valve, due to the unavailability of a 29 mm SAPIEN XT THV mounted on a NovaFlex+ delivery catheter (Edwards Lifesciences) at that time in our institution.
On arrival in the catheterisation laboratory, the patient went into cardiac arrest following ventricular fibrillation. After successful resuscitation, a venoarterial extracorporeal membrane oxygenation (ECMO) was placed through the left femoral vein and artery.
A 12 Fr sheath was placed in the right femoral vein and transseptal puncture was performed under fluoroscopic and TOE guidance (Figure 2A). The atrial septum was dilated with a 14 mm balloon (Figure 2B). Then, the mitral prosthesis was crossed, with difficulty, with a 7 Fr balloon wedge pressure catheter placed within an 8 Fr Mullins™ transseptal sheath (Medtronic, Minneapolis, MN, USA) (Figure 2C). A pre-shaped 0.035” Lunderquist® Extra Stiff guidewire (Cook Medical Inc., Bloomington, IN, USA) was placed at the apex of the left ventricle (Figure 2D). Due to the difficulty in crossing the mitral bioprosthesis, balloon valvuloplasty was performed with an 18 mm balloon (Figure 2E), which resulted in severe prosthetic regurgitation on TOE. The 12 Fr sheath was then exchanged for an 18 Fr expandable sheath (Edwards Lifesciences) with the tip placed in the inferior vena cava. Next, a 26 mm SAPIEN XT THV reversely mounted on a NovaFlex+ delivery catheter was advanced into the right atrium. However, the valve would not go across the atrial septum (Figure 2F, Moving image 2). Therefore, the valve was pulled back, but it was not possible to retract it into the sheath. Consequently, the THV, the delivery catheter and the sheath had to be pulled out as a whole, and the valve had to be explanted with pliers, causing laceration of the right femoral vein with severe bleeding requiring continuous manual compression thereafter, despite the insertion of another 18 Fr expandable sheath. Following an additional dilatation of the atrial septum with an 18 mm balloon (Figure 2G), it was possible to cross the atrial septum with another 26 mm SAPIEN XT THV. Then, with maximum unloading from the ECMO and without rapid pacing, the valve was successfully implanted in the mitral prosthesis (Figure 2H). While the fluoroscopic result appeared satisfactory (Figure 3A), TOE showed a severe mitral regurgitation, which appeared to be mostly centrovalvular but also in part paravalvular (Figure 3B, Moving image 3). It was therefore decided to implant a second prosthesis within the first THV. However, during the preparation of the second valve, the THV that had just been implanted migrated into the left atrium (LA) on the guidewire (Figure 3C, Figure 3D). An attempt to push the valve back into the mitral bioprosthesis with an inflated balloon catheter was unsuccessful. The decision was taken to implant the new THV in the mitral bioprosthesis with one additional ml of contrast medium in the inflation device. This was performed with the migrated prosthesis still on the guidewire (Figure 3E). TOE showed only mild paravalvular regurgitation after the implantation of the second THV, with a mean gradient of 3 mmHg. At this point, it was felt that nothing more could be done for the migrated valve, so the guidewire was pulled out. The migrated THV could be seen moving around freely like a “pinball” in the LA (Figure 3F, Moving image 4). The right femoral vein was closed surgically. An inventory of the material used in the procedure is reported in Table 1.
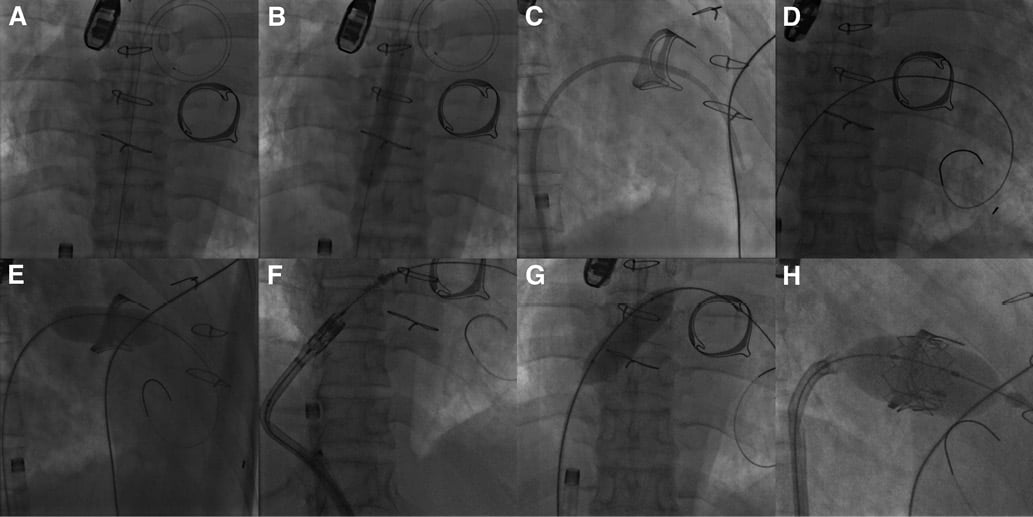
Figure 2. Fluoroscopic views of the emergency transseptal mitral VIV implantation. A) Placement of an Inoue coiled guidewire (Toray Industries, Inc., Tokyo, Japan) in the LA following transseptal puncture. The venous cannula of the ECMO can be seen in the lower part of the picture. B) Dilatation of the atrial septum with a 14 mm balloon. C) Crossing of the mitral bioprosthesis with a balloon wedge pressure catheter placed within a Mullins™ transseptal sheath. D) Placement of a Lunderquist® Extra Stiff guidewire at the apex of the left ventricle. E) Dilatation of the mitral bioprosthesis with an 18 mm balloon. F) Failure to cross the atrial septum with the first THV. G) Additional dilatation of the atrial septum with an 18 mm balloon. H) Implantation of a 26 mm SAPIEN XT valve in the mitral bioprosthesis.
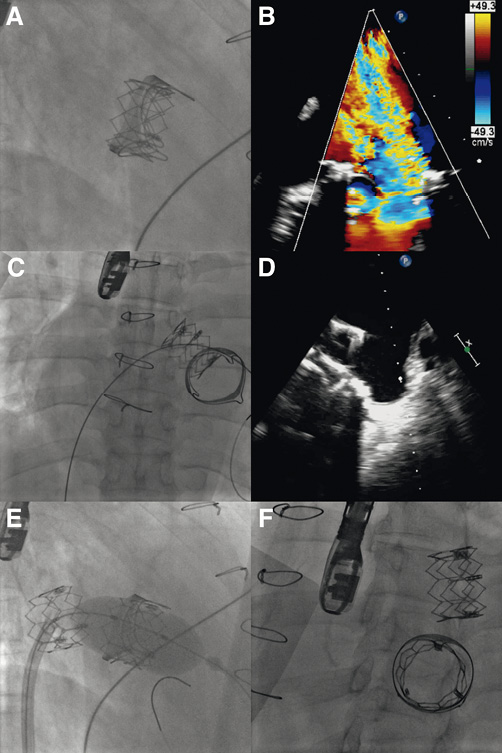
Figure 3. A) Fluoroscopic result of the first THV implanted in the mitral bioprosthesis. B) TOE showing a severe regurgitation of the THV in the mitral position, which appeared to be mostly centrovalvular but also in part paravalvular. Migration of the implanted THV on the Lunderquist Extra Stiff guidewire by fluoroscopy (C), and TOE (D). E) Implantation of the second 26 mm SAPIEN XT valve in the mitral position by fluoroscopy (with the addition of one extra ml of contrast medium in the inflator device in comparison to the first THV). F) Final result following the implantation of the second THV on fluoroscopy. The first THV could be seen moving around freely in the LA.
The subsequent outcome was complicated by gram-negative bacteraemia and sepsis treated with antibiotics in addition to renal failure necessitating temporary dialysis. Furthermore, the patient suffered from heparin-induced thrombocytopenia (HIT) that required the anticoagulation treatment to be switched from unfractionated heparin to danaparoid, which was in turn changed for argatroban following danaparoid cross-reactivity with HIT antibodies. Nevertheless, the overall evolution was favourable, with weaning from mechanical ventilation, inotropic support and ECMO within 10 days of the index procedure. In addition, there was no neurological deficit.
However, on the fourteenth day following the THV implantation, the patient presented an abrupt recurrence of heart failure. This was due to the entrapment of the migrated valve in the implanted valve in a “reverse position”, causing a mean transprosthetic gradient of 20 mmHg and therefore seriously impairing antegrade flow (Figure 4A-Figure 4C; Moving image 5 and Moving image 6). The patient was immediately taken to the catheterisation laboratory. From a right femoral venous access, a pigtail catheter within a Mullins sheath was placed in the LA through the residual atrial septal defect that had been created during the first procedure, and the entrapped valve was successfully dislodged from the implanted valve (Figure 4D). Once more, the migrated valve was moving around freely in the LA.
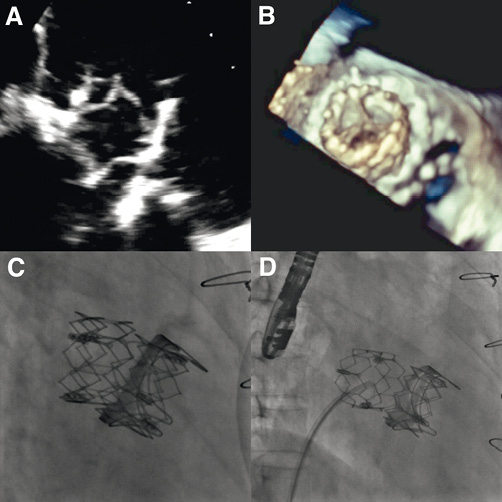
Figure 4. Entrapment of the migrated THV in the implanted valve in a “reverse position” by two-dimensional TOE (A), by 3-D TOE (B), and by fluoroscopy (C). D) Successful dislodgement of the entrapped valve by fluoroscopy using a pigtail catheter through a Mullins sheath.
The second procedure was also followed by an immediate favourable clinical outcome. However, there was great concern that the migrated valve could get entrapped in the implanted valve once more. Therefore, the Heart Team reluctantly planned a bail-out surgical removal of the migrated valve through a left thoracotomy. Such an intervention was deemed as extremely high risk due to the previous interventions and, most importantly, to the requirement for bivalirudin as an anticoagulant during cardiopulmonary bypass because of HIT. However, the day before the planned operation, transthoracic echocardiography was performed, where the migrated valve could not be seen moving around in the LA anymore, and was not entrapped in the implanted valve. Fluoroscopy showed that the valve was not moving around anymore and appeared to be entrapped somewhere in the LA (Figure 5A, Figure 5B, Moving image 7). TOE confirmed the entrapment of the migrated valve in the left atrial appendage (LAA), which was extremely dilated in this patient and large enough to fit the THV (Figure 5C, Figure 5D, Moving image 8). The surgical procedure was therefore called off.
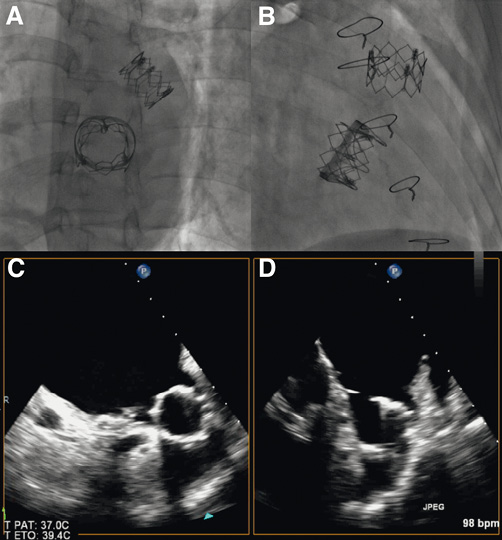
Figure 5. A) and B) Fluoroscopic views showing the migrated valve appearing to be trapped somewhere in the LA. C) and D), TOE showing the migrated valve entrapped in the LAA.
During the following two weeks, there was complete resolution of heart failure signs, and the patient had NYHA Class 2 dyspnoea. Transthoracic echocardiography was repeated, confirming the persistence of the migrated valve in the LAA. The mean transmitral gradient was 9 mmHg, and there was a grade II paravalvular mitral regurgitation. It was decided not to propose any other surgical intervention, to which the patient was in any case opposed. She was discharged 45 days following the index procedure and was able to return home. There were no changes of signs or symptoms of heart failure during the following months, but anticoagulation therapy was stopped after three months due to poor compliance and a spontaneously prolonged prothrombin time. Six months after the index procedure, the patient suddenly died while at home. Autopsy was not performed and the cause of death was undetermined.
Discussion
We report the first emergency implantation of a THV in the mitral position from a transseptal approach. Van Garsse et al recently reported the case of a patient who successfully underwent emergency mitral VIV implantation via a transapical access9. In our patient, a transapical route may have provided a more direct access to the mitral bioprosthesis and allowed an easier positioning of the valve as well as the use of a larger THV. However, it was considered that the advantages presented by such an approach would have been outweighed by the haemorrhagic risk, which we considered to be extremely high in this patient with five prior cardiac surgeries, shock, hepatic failure and DIC.
Therefore, we believed that a transseptal approach would be safer in terms of access site complications. Consequently, due to the unavailability of a 29 mm SAPIEN XT valve mounted on a NovaFlex+ catheter at that time in our institution and to the urgency of the patient’s condition, we decided to attempt the transseptal implantation of a 26 mm SAPIEN XT valve, which was the largest THV that could be delivered through this approach. It was anticipated that this smaller valve could be somewhat undersized in terms of diameter with a risk of migration, though there was a theoretical possibility that the THV deployed at its maximal diameter would remain impacted in the bioprosthesis when taking into account the annulus diameter assessed by 3-D TOE (25.5 mm). This was confirmed by the successful implantation of the second valve with the addition of an extra ml of contrast medium in the inflation device after the failed implantation of the first prosthesis that migrated into the LA. Moreover, the residual paravalvular regurgitation following the implantation of the second valve also implies that a larger diameter would have been more appropriate. With hindsight, the use of a second valve could have been prevented if the first valve had been prepared with the additional contrast medium.
Furthermore, it could also be argued that sole balloon valvuloplasty of the surgical bioprosthesis could have been attempted in order to offer a temporary bail-out. However, such an intervention, which is discouraged by the current guidelines, carries a high risk of leaflet tear and acute regurgitation (as was the case with our patient), with generally disappointing results11,12. While successful bioprosthesis dilatation has been described occasionally13, these scarce reports probably mask many unpublished therapeutic failures14.
Strategies for management of the migrated valve were limited. Percutaneous extraction of the valve (either through the atrial septum or through the mitral bioprosthesis) was not possible. Although surgical explantation should definitely be considered as the treatment of choice in this situation, such an intervention appeared to be high risk in this case due to HIT, as anticoagulation with bivalirudin during cardiopulmonary bypass would have been required. Such a process, which has proven to be feasible, is complicated and associated with a high risk of bleeding15-17. In view of this, it was felt that avoiding another surgery was preferable in this patient who had already undergone multiple operations with a complicated postoperative course following the last intervention. Alternatively, in retrospect, a percutaneous attempt to advance the THV in the LAA could have been considered following the immediate migration of the valve (e.g., with a biotome through a Mullins sheath).
Nevertheless, after the valve had migrated into the LAA and appeared to be in a stable position during the following two weeks, it was thought that a conservative treatment was probably the best option, taking into account the extreme risk of surgery. However, such a hypothesis remains unproven in view of the patient’s unexplained death at six months, as LAA wall erosion by the valve could be one of the reasons leading to such an outcome.
Another aspect of this case that should be highlighted was the use of ECMO. In recent years, ECMO has been used increasingly in the setting of primary percutaneous coronary intervention for acute myocardial infarction complicated by cardiogenic shock18. Drews et al have also described the use of left ventricular assistance for THV implantation in patients with severely depressed left ventricular function or cardiogenic shock19. In our case, the use of ECMO allowed preservation of a stable haemodynamic status during the intervention. Furthermore, the presence of the venous cannula did not interfere with the transseptal puncture or the rest of the procedure. In addition, it should be noted that in the present case the use of ECMO with maximum unloading allowed the implantation of the THV without the requirement for rapid pacing, which would have necessitated the placement of a transvenous lead through a jugular vein as both femoral veins were already utilised.
Conclusions
The present case shows that transseptal transcatheter mitral VIV implantation is feasible, and that it is possible to perform this procedure in an emergency setting in a patient with cardiogenic shock receiving ECMO. Furthermore, THV migration in the LA is a possible complication, which may lead to late entrapment in a reverse position, with significant haemodynamic consequences.
Conflict of interest statement
D. Himbert is a proctor physician for Edwards Lifesciences and Medtronic Inc. P. Nataf is a consultant for Medtronic Inc., and a proctor for Edwards Lifesciences. A. Vahanian is on the advisory board for Medtronic Inc., and receives speaker’s fees/honoraria from Edwards Lifesciences and Medtronic Inc. The other authors have no conflicts of interest to declare.
Online data supplement
Moving image 1. Left atrial 3-D TOE view of the stenotic mitral bioprosthesis with a nearly complete fusion of the leaflets.
Moving image 2. Fluoroscopy showing failure to cross the atrial septum with the THV.
Moving image 3. TOE showing a severe regurgitation following implantation of the first THV in the mitral position. The leak appeared to be mostly centrovalvular but also in part paravalvular.
Moving image 4. Final result following the implantation of the second THV by fluoroscopy. The first THV could be seen moving around freely in the LA like a “pinball”.
Moving image 5. Entrapment of the migrated THV in the implanted valve in a “reverse position” by two-dimensional TOE with (right) and without (left) colour Doppler.
Moving image 6. Entrapment of the migrated THV in the implanted valve in a “reverse position” by 3-D TOE showing only a limited opening of the entrapped valve during systole.
Moving image 7. Fluoroscopy showing the migrated valve appearing to be trapped somewhere in the LA.
Moving image 8. TOE showing the migrated valve entrapped in the LAA.
Supplementary data
To read the full content of this article, please download the PDF.
Moving image 1. Left atrial 3-D TOE view of the stenotic mitral bioprosthesis with a nearly complete fusion of the leaflets.
Moving image 2. Fluoroscopy showing failure to cross the atrial septum with the THV.
Moving image 3. TOE showing a severe regurgitation following implantation of the first THV in the mitral position. The leak appeared to be mostly centrovalvular but also in part paravalvular.
Moving image 4. Final result following the implantation of the second THV by fluoroscopy. The first THV could be seen moving around freely in the LA like a pinball.
Moving image 5. Entrapment of the migrated THV in the implanted valve in a reverse position by two-dimensional TOE with (right) and without (left) colour Doppler.
Moving image 6. Entrapment of the migrated THV in the implanted valve in a reverse position by 3-D TOE showing only a limited opening of the entrapped valve during systole.
Moving image 7. Fluoroscopy showing the migrated valve appearing to be trapped somewhere in the LA.
Moving image 8. TOE showing the migrated valve entrapped in the LAA.

