
CASE SUMMARY
BACKGROUND: A patient with severe functional mitral valve regurgitation and severe coronary artery disease with high surgical risk underwent a two-step strategy with CABG first, follow-up monitoring of the MR, and MitraClip implantation as a second step if MR did not improve sufficiently.
INVESTIGATION: During the MitraClip procedure an acute oxygen desaturation occurred. Echocardiography revealed that a rupture of the interatrial septum had occurred with a following severe right-to-left shunt. The different treatment options of fast track procedure, or establishing stable conditions for a controlled MitraClip implantation, or to finish the procedure were discussed.
DIAGNOSIS: Rupture of the interatrial septum with haemodynamically relevant right-to-left shunt during MitraClip implantation.
MANAGEMENT: Temporary veno-venous extracorporeal membrane oxygenation (ECMO) support to proceed with the MitraClip procedure under stable conditions and final atrial septal defect closure was performed.
KEYWORDS: atrial septal defect, interatrial rupture, MitraClip, veno-venous extracorporeal membrane oxygenation (ECMO)
PRESENTATION OF THE CASE
A 61-year-old male patient with severe functional mitral regurgitation (MR) and severe coronary artery disease with high surgical risk for CABG with combined surgical mitral valve repair or replacement (logistic EuroSCORE 27.6%, EuroSCORE II 11.6%) underwent a two-step strategy (due to the institutional Heart Team decision) with coronary artery bypass grafting (CABG) (on-pump beating heart) first, follow-up monitoring of the MR, and MitraClip® (Abbott Vascular Inc., Santa Clara, CA, USA) implantation as a second step if MR did not improve sufficiently.
Three months after CABG, the patient presented at our clinic with dyspnoea of New York Heart Association functional Class IV. Echocardiography still showed severe functional MR. Right heart function was slightly reduced (tricuspid annular plane systolic excursion 12 mm).
For the MitraClip procedure, the patient was mechanically ventilated and was under general anaesthesia. Haemodynamic measurements showed an increased right atrial pressure of 23 mmHg, while left atrial pressure was only 12 mmHg (V-wave 18 mmHg). Cardiac output was 2.2 l/min, cardiac index 1.2 l/min/m2, pulmonary wedge pressure 27 mmHg (V-wave 33 mmHg), pulmonary artery pressure 40/19 (mean 28) mmHg. Before transseptal puncture, bulging of the atrial septum into the left atrium was noticed (Figure 1).
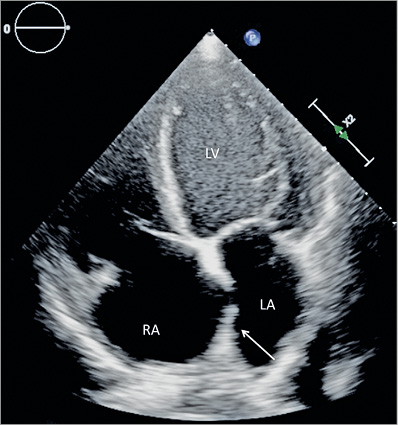
Figure 1. TEE four-chamber view: bulging of the interatrial septum into left atrium (arrow) prior to transseptal puncture.
Transseptal puncture at 4.6 cm above the annulus as well as insertion of the steerable guide catheter over an Amplatz™ super-stiff wire, 7 cm J-tip (Boston Scientific, Marlborough, MA, USA) was performed without any problems. During positioning of the MitraClip, height of the clip delivery system was lost due to rupture of the atrial septum (Figure 2) so that positioning was complicated, but a central grasping of the anterior and posterior mitral valve leaflets was successfully performed. However, residual MR was still moderate, with regurgitant jets lateral and medial to the clip. Then, arterial oxygen saturation dropped suddenly to 75% under ventilation with 100% oxygen.
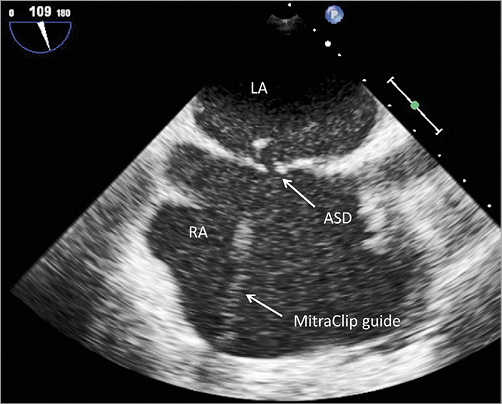
Figure 2. 2D TEE: view of the interatrial septum with an atrial septal defect (ASD) next to the MitraClip guide.
Echocardiography revealed a significant interatrial right-to-left shunt next to the guide catheter. Since positioning of the MitraClip was complicated and the result of the first grasp was not satisfactory, a short discussion for a fast track procedure with a potentially suboptimal result of the MR was ruled out. Haemodynamic stability was persistent the whole time but reduced saturation under ventilation of 100% oxygen was present.
How would I treat?
THE INVITED EXPERT’S OPINION

It would be remiss not to begin by mentioning that the baseline haemodynamics provided are a little perplexing. A left atrial pressure of 12 mmHg in a patient with severe functional mitral regurgitation (MR) and presumably significant left ventricular (LV) dysfunction (history of functional MR, NYHA Class IV symptoms, and a cardiac index of 1.2 L/min/m²) is hard to understand. Nevertheless, we are faced with a patient with apparent severe hypoxia due to right-to-left shunting across a tear in the interatrial septum caused by trauma from the MitraClip delivery system during the manoeuvres to deploy a first MitraClip. In addition, the operators feel compelled to add one or more additional clips to treat residual moderate MR (residual MR medial and lateral to the first MitraClip), which seems reasonable given that the patient has already undergone coronary artery bypass surgery three months previously.
My initial response would be to try medical manoeuvres to raise the left atrial pressure in an effort to minimise the degree of right-to-left shunting. This could be carried out with the administration of a fluid bolus (500 cc to 1,000 cc) and IV pressors (e.g., phenylephrine, adrenaline, noradrenaline). The choice of pressor would depend on the left ventricular systolic function, with adrenaline being favoured particularly in the presence of significant LV systolic dysfunction. I would probably try to achieve a systolic blood pressure of ~150 mmHg.
If this intervention were not sufficient, I would gain an additional common femoral venous access (10 Fr), deliver an ES Amplatz wire into the left upper pulmonary vein, and inflate an atrial septal defect sizing balloon beside the MitraClip guide. Given the size of the defect shown on the echo image, my suspicion is that an AMPLATZER™ 24 mm sizing balloon (St. Jude Medical, St. Paul, MN, USA) should be sufficient. My concern about this sizing balloon is that it might physically interfere with manipulations of the MitraClip and its delivery system in the left atrium. If this were the case, then the wire stabilising the sizing balloon could be repositioned into the right upper pulmonary vein, which should make these manipulations easier.
Given the degree of right-to-left shunting during the case, I would anticipate that the traumatic defect in the interatrial septum should be closed with an atrial septal defect (ASD) occluder device at the completion of the case. Under normal circumstances in a patient with long-standing mitral regurgitation and left ventricular dysfunction, I would probably favour the use of a fenestrated ASD occluder (Occlutech®; Occlutech, Helsingborg, Sweden) to facilitate the off-loading of the left atrium from high left atrial pressures. The latter devices require a special order and would not typically be on the shelf in the catheterisation laboratory and so would have to be implanted in a staged manner. However, in this case, where the left atrial pressure for some perplexing reason was quite normal at baseline, the use of a conventional ASD occluder device is probably appropriate.
Conflict of interest statement
The author has no conflicts of interest to declare.
How would I treat?
THE INVITED EXPERTS’ OPINION

Schmidt et al present an interesting clinical scenario, namely a 61-year-old man with severe functional mitral regurgitation (MR) and New York Heart Association Class IV symptoms who was referred for percutaneous edge-to-edge mitral valve repair (MitraClip). Pre-procedure, the patient was noted to have severe right atrial (RA) hypertension, tricuspid regurgitation, elevated pulmonary pressure, right ventricular impairment and decreased cardiac output.
The operators performed a transseptal puncture at the recommended height. They delivered one clip in a central position with residual moderate MR, but manipulation of the MitraClip sheath was complicated by a rupture of the atrial septum, leading to a significant right-to-left shunt with severe hypoxia despite ventilation with 100% oxygen.
There are four questions to be addressed. Firstly, is the ruptured interatrial septum likely to be responsible for this patient’s deterioration? Secondly, do we accept the result of the MitraClip (reduction only to moderate MR) or attempt to deliver further clips? Thirdly, how do we size the defect accurately? Finally, what device do we use to close the defect?
Firstly, in this case the patient had significant right atrial hypertension that has resulted in a right-to-left shunt through the interatrial septum around the delivery sheath. As the patient has become hypoxic despite an FiO2 of 100%, we feel it is clear that the shunt is responsible for the hypoxia.
Secondly, there has been a reduction in the MR with the placement of one clip. It was a complicated and difficult procedure so we would accept the result and not attempt to reduce the residual MR further with additional clips, but focus on closing the iatrogenic ASD percutaneously.
Thirdly, to estimate congenital ASD size, our standard practice is to use a combination of a sizing balloon and echo assessment. In this case, we would inflate a sizing balloon cautiously for fear of increasing the iatrogenic ASD size. The sizing balloon could be advanced over a stiff wire with the tip in a pulmonary vein after withdrawal of the MitraClip delivery system, and inflated under transoesophageal echocardiography (TOE) guidance just until the shunt disappears, thereby providing confirmation that the iatrogenic ASD is responsible for the hypoxia.
Finally, we would normally use a self-centring occluder device sized according to the waist of the sizing balloon, e.g., AMPLATZER™ Septal Occluder (ASO) (St. Jude Medical). However, in this case we know that the RA pressure is severely elevated and the atrial septum is bowed to the left. The ASO device has only a 5 mm rim on the RA side, so a 10 mm device has only a 20 mm RA disc. There is therefore an increased risk of device embolisation to the systemic side. It might therefore be safer to use a non-self-centring device, e.g., a 25 mm AMPLATZER™ PFO or Cribriform Occluder (St. Jude Medical) or a 30 mm GORE® CARDIOFORM Septal Occluder (W.L. Gore & Associates, Flagstaff, AZ, USA).
In summary, we would accept the reduction to moderate MR and close the iatrogenic ASD with a symmetrical non-self-centring device, probably a 25 mm AMPLATZER Cribriform Occluder.
Conflict of interest statement
D. Northridge has consulted and proctored for St Jude Medical. The other authors have no conflicts of interest to declare.
How did I treat?
ACTUAL TREATMENT AND MANAGEMENT OF THE CASE
Treatment options
The treatment options in the acute situation were on the one hand a fast track procedure with an unsatisfactory result for the MR or, on the other hand, establishing stable conditions to perform MitraClip implantation in the standard way. Another option was to end the procedure with only acute closure of the ASD and to discuss medical treatment or repeat surgery for the MR.
Implantation of the MitraClip is a percutaneous treatment option available for high-risk patients. The EVEREST I and II trials demonstrated the safety and efficacy of MitraClip therapy1. The MitraClip procedure is performed using transfemoral/transseptal access to the left atrium. The guide catheter has a size of 22 Fr (7.33 mm) when crossing the atrial septum. An ASD may occur following transseptal puncture. Smith et al reported a prevalence of 27% of ASD after MitraClip procedures2. The prevalence of MR severity ≤2+ at 12 months has been described as being significantly lower in ASD patients than in patients without ASD2. Schueler et al even described a 50% rate of persisting ASD at six months after MitraClip implantation. In that study, interatrial shunting was associated with worse clinical outcomes and increased mortality rates3. In the EVEREST II trial, the incidence of MitraClip-induced ASD requiring intervention was 2.2%1.
Guidelines for classification of the ASD and the need for ASD closure do not exist. Closure of an ASD is performed in cases of haemodynamic instability or an acute drop in oxygen saturation4.
Veno-venous ECMO can be performed to replace the function of the lung (oxygenation) or veno-arterial ECMO to replace heart and lung (circulation and oxygenation). Both options can be set up percutaneously.
Veno-venous ECMO support
Veno-venous ECMO support with 17 Fr access into the superior vena cava via the right jugular vein and 25 Fr access into the inferior vena cava via the left femoral vein were initiated in order to proceed with the MitraClip procedure under stable conditions (Figure 3). With ECMO flow of 3 l/min, oxygen saturation rose to 100% immediately. The MitraClip procedure was continued with implantation of two clips and reduction of the MR to trace. Then, veno-venous ECMO support was reduced to a flow rate of <1 l/min to check oxygen saturation and the need for an ASD occluder. Oxygen saturation dropped immediately to 90%, even with the guide catheter still positioned across the atrial septum so that closure of the atrial septum was necessary.
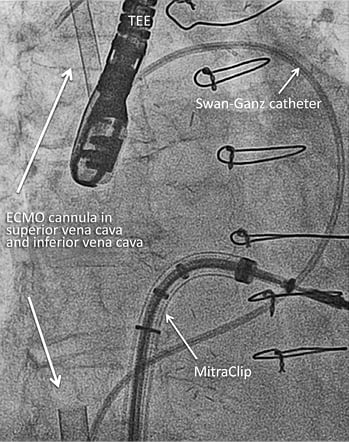
Figure 3. Fluoroscopic view during MitraClip procedure with veno-venous ECMO support.
ASD evaluation
Echocardiographic ASD measurements under stable conditions even with the guide removed were performed due to the ECMO support. The ASD was 19×8 mm² with a shunt area of 1.4 cm2 and a bidirectional right-to-left and left-to-right shunt during veno-venous ECMO support (left-to-right velocity time integral [VTI]: 31.6 cm3, right-to-left VTI: 66.3 cm3) was diagnosed (Figure 4).
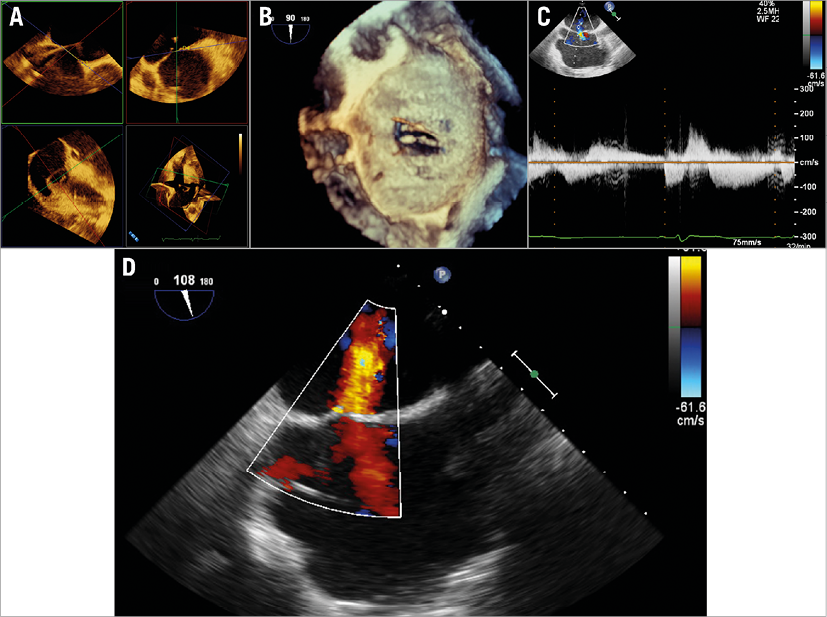
Figure 4. TEE view of the ASD. A) Four planes for measurements of the ASD. B) 3D TEE displays the ASD. C) Continuous wave Doppler measurements of the shunt (during veno-venous ECMO). D) 2D TEE with colour imaging of the ASD.
ASD closure
A 21 mm Occlutech device was implanted and stable oxygen saturation was persistent, even with an ECMO flow of only 0.5 l/min, so that ECMO cannulas were removed and all venous access sites were closed with ProGlide® devices (Abbott Vascular, Santa Clara, CA, USA).
Haemodynamics
Haemodynamics after closure of the ASD and removal of the ECMO showed an increase of about 1 l/min of cardiac output (3.1 l/min; cardiac index 1.76 l/min/m2) and reduction of right atrial pressure to 11 mmHg, pulmonary wedge pressure to 15 mmHg (V-wave 22 mmHg) and pulmonary artery pressures of 51/14 (mean 25) mmHg.
Post-procedure and MR/ASD evaluation
On day two after the procedure, the patient was transferred to the regular ward and mobilised without any difficulties. Follow-up echocardiography at day 9 still showed trace MR with good leaflet insertion and a mean transmitral pressure gradient of 3 mmHg and no ASD.
Conclusion
An elegant way to treat an iatrogenic ASD with right-to-left shunt during a MitraClip procedure with acute reduction of the arterial saturation is the procedural use of veno-venous ECMO for stable conditions to achieve a good result for the mitral valve regurgitation and the closure of the ASD (Figure 5).
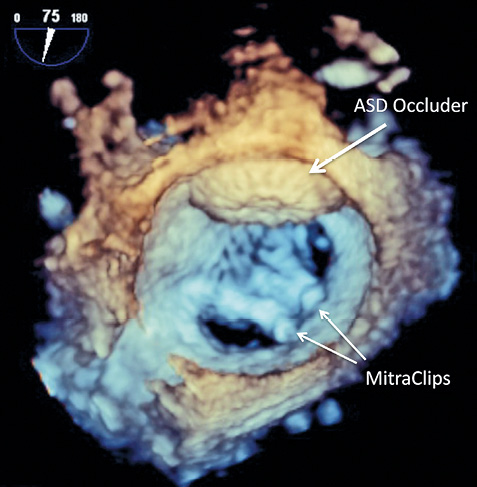
Figure 5. 3D TEE view of the final result after implantation of two MitraClips and closure of the ASD.
Conflict of interest statement
K-H. Kuck is a consultant for Abbott Vascular. C. Frerker receives lecture honoraria from Abbott Vascular. T. Spangenberg received lecture honoraria from Maquet. The other authors have no conflicts of interest to declare.

