
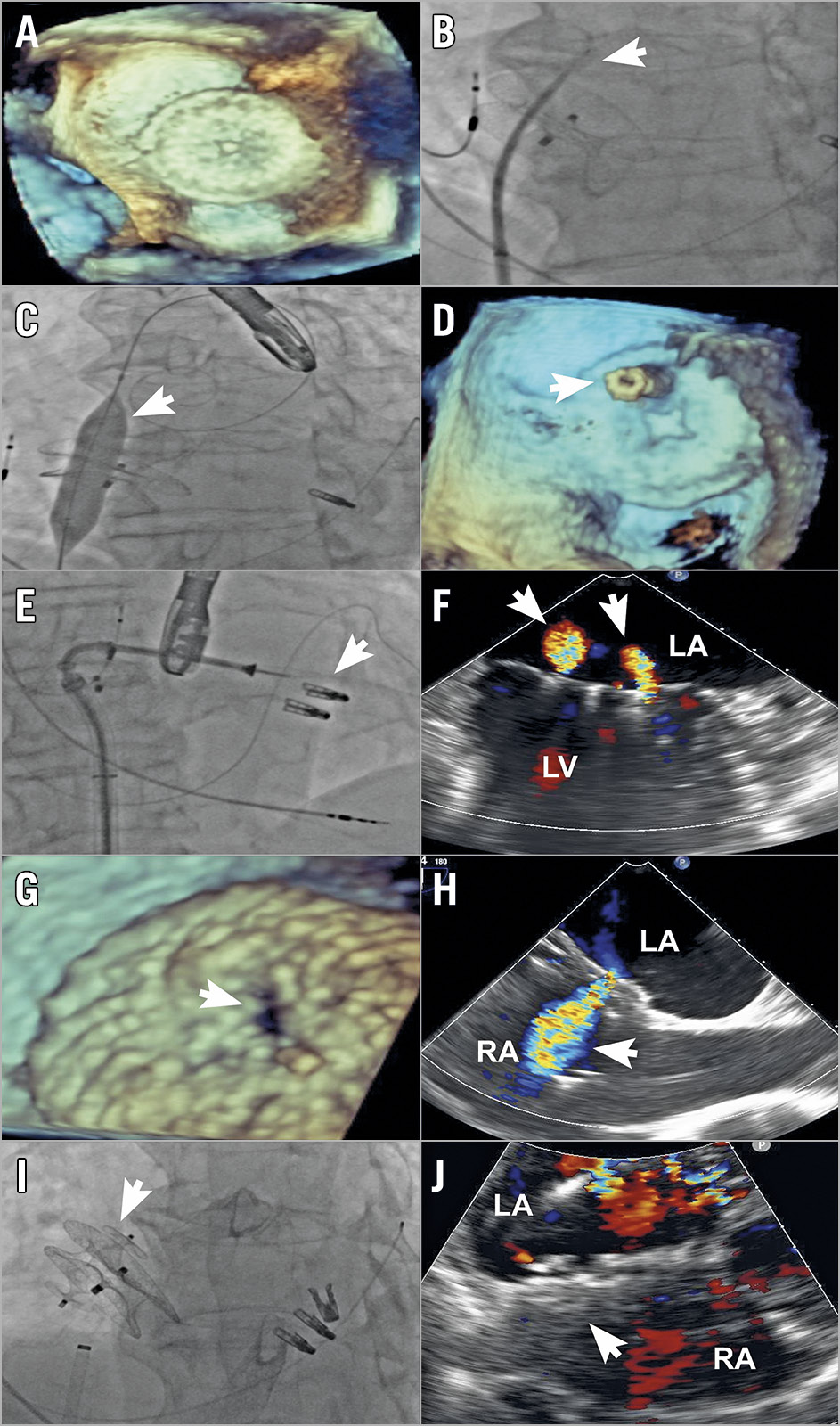
Figure 1. Transcatheter mitral valve repair after prior MitraClip therapy and atrial septal defect closure. A) Transoesophageal echocardiography (TEE) showing prior atrial septal occluder (ASO) and no space posterior to the ASO for safe transseptal puncture. B) Fluoroscopy showing a transseptal puncture through an ASO (arrow). C) Fluoroscopy showing predilatation with 12 × 40 mm balloon (arrow). D) TEE with three-dimensional (3D) imaging shows a steerable guide catheter through the ASO (arrow). E) Fluoroscopy showing deployment of an additional clip (arrow) through the ASO. F) TEE showing trivial MR (arrows) after additional clips. G) 3D-TEE showing new iatrogenic defect (arrow). H) TEE showing residual shunting (arrow). I) Fluoroscopy showing placement of the AMPLATZER Vascular Plug II (arrow). J) Transthoracic echocardiography showing no residual shunting (arrow). LA: left atrium; LV: left ventricle; RA: right atrium
An 83-year-old man presented with symptomatic, recurrent severe mitral regurgitation (MR) two years after a previous transcatheter mitral valve repair with the MitraClip® device (Abbott Vascular, Santa Clara, CA, USA), in which closure of a large iatrogenic atrial septal defect (iASD) with a 28 mm AMPLATZER™ atrial septal occluder (ASO; Abbott Vascular) was performed. Following a multidisciplinary Heart Team evaluation, a second MitraClip procedure was undertaken.
Initial procedural imaging demonstrated no space posterior to the ASO for a safe transseptal puncture, necessitating crossing of the ASO for the placement of the 24 Fr steerable guide catheter (SGC) to enable the second MitraClip procedure (Figure 1A). Using an NRG® C0 Transseptal Needle (Baylis Medical, Toronto, ON, Canada) with 10 watts of radiofrequency (RF) energy and an 8 Fr Mullins sheath, a crossing location on the ASO with a height of 4.5 cm to the mitral valve was utilised (Figure 1B). A 230 cm Inoue wire (Toray, Tokyo, Japan) was inserted into the Mullins sheath, followed by balloon dilatation of the ASO occluder segment in escalating sizes. However, the SGC was unable to pass across the ASO until dilatation with a 12×40 mm Atlas® GOLD balloon (Bard, Tempe, AZ, USA) and passage of an Inoue dilator (Toray) (Figure 1C, Figure 1D). Transcatheter repair with two additional MitraClip NTR clips was performed, leading to significant reduction in MR (Figure 1E, Figure 1F). Although a new iatrogenic defect was created through the previous ASO, this was not closed initially due to the possible need for additional MitraClip therapy, as well as the potential for unloading of left atrial hypertension (Figure 1G, Figure 1H).
The patient returned one month later with heart failure (NYHA Class III) symptoms. A 14 mm AMPLATZER™ Vascular Plug II (AVP II; Abbott Vascular) was used to close the new iASD completely using intracardiac echocardiography and fluoroscopic guidance, leading to clinical improvement (Figure 1I, Figure 1J). The MR reduction remained stable (grade 1+), and the patient had only mild dyspnoea (NYHA Class II) at one-year follow-up. Also, the AVP II was well seated with a minimal residual shunt and the patient was without haemolytic anaemia despite continued anticoagulation therapy.
To our knowledge, the present case represents the first experience of successful transcatheter therapy for residual MR after MitraClip therapy and ASD closure. These techniques may also be applicable for other left atrial interventions (e.g., appendage closure, pulmonary vein isolation) that may be required after atrial septal closure. While closure of an ASD with occluder devices can pose challenges for repeat access to the left atrium, this case demonstrates the feasibility of crossing such devices to perform beneficial therapy.
Conflict of interest statement
R. Bae reports being on the speakers’ bureau of Abbott Vascular. P. Sorajja has received research grants and consulting fees from and is on the speakers bureau of Edwards Lifesciences, Medtronic, Boston Scientific, and Abbott Structural, has equity in and is a consultant for Admedus, and has received consulting fees from Gore and Cardionomic. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

