
Transcatheter mitral valve repair by leaflet approximation using the MitraClip® (Abbott Vascular, Santa Clara, CA, USA) is an established treatment of selected patients with either primary or secondary mitral regurgitation (MR). Although effective in the majority of cases, there are instances of residual or recurrent MR due to gaps between clips, residual pathology on the side of the clip or, rarely, due to leaflet perforation. These situations may not be amenable to treatment with an additional clip. The presence of a clip-induced tissue bridge makes treatment with a transcatheter valve replacement challenging. Surgical repair or replacement, though possible, is challenging since most of the patients are inherently high/prohibitive risk for traditional surgical repair/replacement. In selected patients, the residual/recurrent MR can be treated by placement of vascular occluders or plugs between clips, on the side of the clip or at the site of perforation. The procedure is generally safe and effective. However, inadequate reduction of MR, haemolysis, or stenosis may result. The AMPLATZER family of devices comprises occluders of different shapes, sizes and nitinol mesh architecture. The AMPLATZER™ Duct Occluder II (ADO II), and AMPLATZER™ Vascular Plugs (AVP) (both St. Jude Medical, St. Paul, MN, USA) are made of tightly woven nitinol mesh and therefore associated with less intravascular haemolysis (Figure 1). Similarly, the GORE® Septal Occluder (GSO; W.L. Gore & Associates, Inc., Flagstaff, AZ, USA) comprises a nitinol frame covered with expanded polytetrafluoroethylene (ePTFE) which is occlusive and not associated with haemolysis (Figure 1). The traditional atrial and ventricular septal occluders are made of loosely bound nitinol mesh and can be associated with intravascular haemolysis.
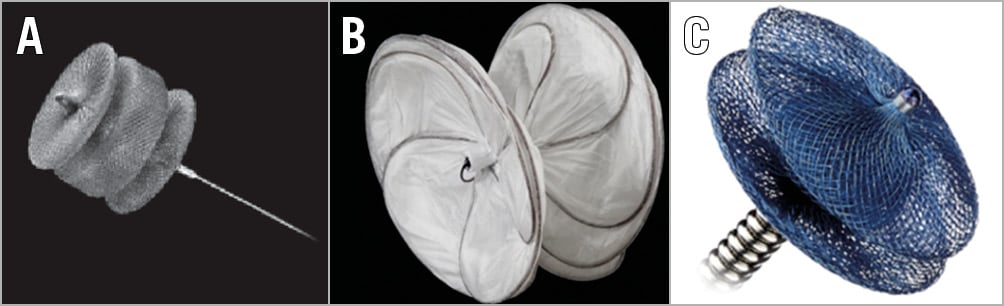
Figure 1. Devices that are used off-label to close residual mitral regurgitation in association with a MitraClip. A) AMPLATZER Vascular Plug II. B) GORE Septal Occluder. C) AMPLATZER Duct Occluder II. Both the AMPLATZER Vascular Plug and Duct Occluder II are made of tightly woven nitinol mesh. The GORE Septal Occluder is a nitinol frame covered with expanded polytetrafluoroethylene (ePTFE). Both tightly woven nitinol mesh and ePTFE are associated with a low risk of intravascular haemolysis.
In this edition of EuroIntervention, Niikura and co-authors describe the outcome and follow-up of nine patients who underwent treatment of residual or recurrent MR, due to interclip and paraclip residual MR or leaflet perforation using the AMPLATZER Vascular Plug II (AVP II)1.
Over a period of four years, the authors found nine cases of significant MR following MitraClip implantation that could be treated with transcatheter off-label placement of the AVP II. There were cases of leaflet perforation and interclip and paraclip MR. The AVP II consists of two discs interconnected by a lobe. The authors found that deployment of one disc and the lobe on the ventricular side and deployment of the proximal disc on the atrial side of the leaflet coaptation line was most effective in reducing MR. The size of the device chosen to close the defect was around 30 to 40% larger than the defect size. No atrial or ventricular septal occluders, ADO II or GSO were used in this study. As a result of a thoughtful sizing algorithm and excellent procedural technique, there was significant reduction of MR, no device embolisation and no intravascular haemolysis. Based on this moderately sized experience, the authors concluded that the AVP II plug was most suitable for closure of such defects.
Treatment of MR associated with MitraClip using occluders or plugs has been described previously. In our series we closed cases of interclip and paraclip residual MR using the ADO II2. The ADO II plug consists of two discs which are 6 mm larger than the device waist and made of very fine micro-nitinol which can be deployed through a 6 Fr (or smaller) multipurpose guide catheter. In our series there was no evidence of haemolysis, although the size of defect that could be closed was limited by the available size of the ADO II. The largest size waist and disc of the ADO II is 6 and 12 mm, respectively, limiting the size of the defect that can be closed with this device without risking device embolisation.
The use of AMPLATZER atrial and ventricular septal occluders for the treatment of residual MR is effective for large defects3. The two main disadvantages of this method are the need for a larger size delivery sheath and the potential for significant intravascular haemolysis (Singh GD. My Toughest MitraClip Case: Peri-mitraclip Regurgitation Treated With Amplatzer Septal Occluder Resulting in Severe Hemolysis Treated With Snare Retrieval. Presented at TVT 2017, Chicago, IL, USA, 16 June 2017).
The GSO, which is made of occlusive PTFE, has been described in the treatment of large paraclip leaks, which were initially closed with a ventricular septal occluder that had caused severe haemolysis4. The ventricular septal defect (VSD) occluder was snared and removed, then successfully replaced with a GSO device, with complete resolution of haemolysis. The main disadvantage of the GSO device is that it requires a 65 cm long 10 Fr sheath to deliver the device and can be difficult to navigate through the defect.
The AVP II consists of two discs of equal size and an interconnecting lobe. Given that the direction of blood flow is along a pressure gradient between the left ventricle and left atrium, it stands to reason that placement of one disc and the lobe below the line of coaptation on the ventricular aspect provides a greater reduction of MR and improved device stability (Figure 2). The availability of a broad range of sizes, low device profile and tightly woven nitinol architecture make the AVP II device ideally suited for the purpose described by Niikura and colleagues, similar to that of treatment of paravalvular leaks following surgical valve replacement5. The absence of haemolysis in this small series is encouraging and should be evaluated in a larger cohort of patients.
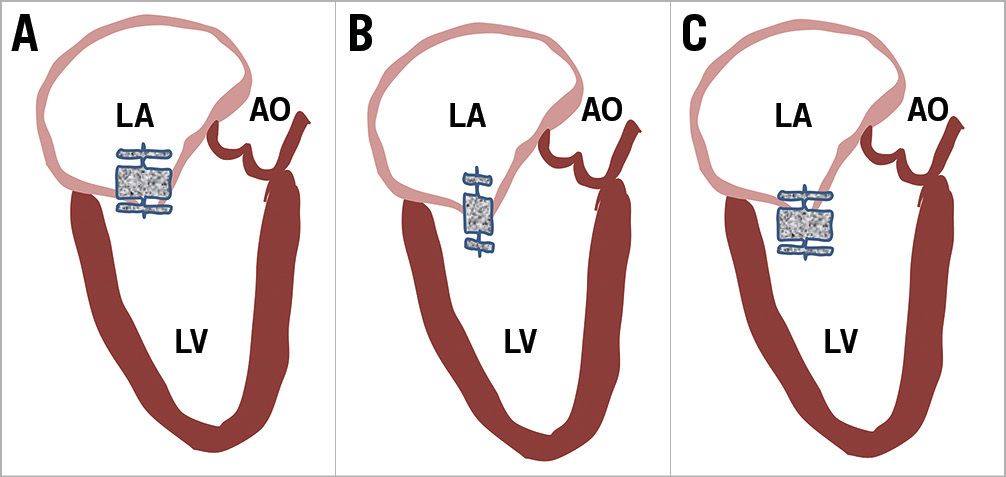
Figure 2. Different locations of deploying the AMPLATZER Vascular Plug II. A) Placement of the plug with only the distal lobe on the ventricular side of the mitral valve. This position has a high chance of embolisation as well as inadequate reduction of regurgitation. B) Placement of the plug with the middle lobe at the level of the mitral valve causes stenting to open the gap, and a high chance of embolisation. C) Placement of two lobes on the ventricular side of the mitral valve results in the best closure rate and lowest risk of embolisation. AO: aorta; LA: left atrium; LV: left ventricle
In summary, in the current era of transcatheter mitral valve repair, closure of interclip or paraclip regurgitation or leaflet perforation leading to recurrent/residual MR can be treated by placement of an AVP II, in a method similar to treatment of surgical paravalvular leaks. The procedure is safe, effective and can be associated with a low incidence of device embolisation and haemolysis. Further evaluation in a larger cohort of patients is eagerly anticipated.
Conflict of interest statement
S. Kar reports receiving grants from Abbott Vascular, Boston Scientific and Edwards Lifesciences. R. Sharma reports receiving grants from Abbott Vascular, Boston Scientific, Medtronic and Edwards Lifesciences.

