CASE SUMMARY
BACKGROUND: A 70-year-old man was admitted to the intensive care unit of our hospital suffering from acute decompensated heart failure (NYHA functional Class IV) due to a severe ischaemic cardiomyopathy. A baseline transthoracic echocardiography showed a severe left ventricle systolic dysfunction with a left ventricle ejection fraction of 15% and a severe mitral regurgitation. Due to the high surgical risk, the patient was deemed inoperable by the Heart Team and it was not possible to wean him from inotropic support because of the consequent sudden deterioration of vital signs.
INVESTIGATION: Laboratory test, transthoracic echocardiography, transoesophageal echocardiography.
DIAGNOSIS: Acute decompensated heart failure in a patient with severe mitral regurgitation deemed inoperable by the Heart Team.
MANAGEMENT: Percutaneous mitral valve repair with MitraClip system with elective circulatory support carried out by the Impella® system.
KEYWORDS: heart failure, mitral regurgitation, percutaneous mitral valve repair
PRESENTATION OF THE CASE
A 70-year-old man was admitted to the intensive care unit of our hospital suffering from acute decompensated heart failure (NYHA functional Class IV) due to severe ischaemic cardiomyopathy. The patient had previously undergone coronary artery bypass surgery, implantation of an implantable cardioverter-defibrillator (ICD), and cardiac resynchronisation therapy. At admission, the patient was tachypnoeic, the heart rate was 95 bpm, and systemic blood pressure was 90/60 mmHg. Baseline kidney function was depressed (estimated glomerular filtration rate of 25 mL/min/1.73 m2) and urine flow rate was <50 ml/hr. Baseline brain natriuretic peptide (BNP) value was 1,003 pg/mL. Medical recompensation with oxygen therapy, vasodilators and diuretics was immediately started. Intensive intravenous inotropic support was initiated, including dobutamine (10 µg/kg/min) and levosimendan (0.1 µg/kg/min). A baseline transthoracic echocardiography (TTE) showed a severe left ventricle (LV) systolic dysfunction with an LV ejection fraction (LVEF) of 15% and a severe mitral regurgitation (MR) with a convergence area of the regurgitant flow involving the central part of the valve (A2-P2). Baseline echocardiographic parameters are depicted in Table 1. The transoesophageal echocardiogram (TEE) confirmed the severity of the MR and clarified its mechanisms which consisted of annular dilatation, tethering of the posterior mitral leaflet and pseudo-prolapse of the anterior mitral leaflet. TEE also showed a severe spontaneous echo contrast (SEC) in the left atrial appendage.
Once a clinical stabilisation had been achieved, the patient underwent a coronary angiography that showed the patency of the bypass grafts and the absence of significant de novo lesions. Despite the improvements regarding the symptoms and haemodynamics of the patient, it was not possible to wean him from inotropic support because of the consequent sudden deterioration of vital signs. Moreover, due to the high surgical risk (EuroSCORE II: 48.0%, Society of Thoracic Surgeons [STS] score 11.5%), the patient was deemed inoperable by the Heart Team.
How would I treat?
THE INVITED EXPERT’S OPINION
This case represents a patient with no conventional therapy options. Considering new percutaneous options presents two major considerations: what is technically and procedurally feasible, and what is the potential for meaningful clinical benefit.
In my practice in the USA the only option would be MitraClip (Abbott Vascular, Santa Clara, CA, USA) on a compassionate use basis, since only degenerative mitral regurgitation is FDA approved and functional mitral regurgitation is available only as part of the randomised COAPT trial, which excludes EF <20% and haemodynamic instability requiring inotropic support. In many other parts of the world both MitraClip and Carillon (Cardiac Dimensions Inc., Kirkland, WA, USA) are available in practice. The presence of cardiac resynchronisation therapy (CRT) with a coronary sinus lead is not an absolute contraindication to annuloplasty with Carillon, but does make MitraClip attractive1. In addition, there is a greater published experience with MitraClip in patients with severe heart failure2. The central nature of the mitral regurgitation (MR) jet is also attractive for MitraClip. Although annular dilatation and tethering of the posterior mitral leaflet might make grasping difficult, it should be possible, even if a strategy of a first clip to stabilise the leaflets followed by a second clip to optimise MR reduction is needed. An early human effort for percutaneous mitral replacement might also be an option in some centres.
A more difficult question is what expected benefit this patient might derive from mitral repair or replacement. While the language of the FDA approval for MitraClip requires its use for patients “in whom existing comorbidities would not preclude the expected benefit from reduction of the MR”, there is no consensus on how to define this boundary between benefit and futility. This patient has an STS risk of 11.5%, well below the >13% level reported in the EVEREST High Risk Registry, in which clinical benefit was seen. This patient has several poor prognostic factors. The LVEF is only 15%. The pulmonary artery pressure (PAP) is certainly elevated. TAPSE (tricuspid annular plane systolic excursion) >15 mm (an indication of right ventricular [RV] dysfunction), and extreme elevations of pro-BNP have been identified as poor prognostic indicators3. Ultimately, most such predictors characterise populations and are difficult to apply to individual patients.
Ultimately, I would use MitraClip if available. While this patient might undergo percutaneous mitral replacement in some centres, patients with a better medium-term outlook might be better for the early mitral replacement efforts.
Conflict of interest statement
The author is a consultant for Abbott Vascular.
How would I treat?
THE INVITED EXPERT’S OPINION
Functional mitral regurgitation (FMR) occurs when normal or nearly normal mitral leaflets are prevented from proper coaptation by underlying left ventricular (LV) dysfunction, mitral annular dilation, or both. FMR is associated with an adverse prognosis in ischaemic LV dysfunction4.
Treatment of FMR and decompensated heart failure may begin with guideline-directed maximal medical therapy for LV dysfunction and heart failure, including a subtle combination of inotropes and vasodilators and if necessary mechanical unloading (either with intra-aortic balloon counterpulsation or arteriovenous extracorporeal membrane oxygenation [ECMO]). Myocardial revascularisation through PCI should be considered in case of persistent ischaemia in the region of papillary muscle attachment. Finally, mitral valve reconstruction using a specially designed ring to address the tethering of the posterior papillary muscle (for instance, the ETlogix; Edwards Lifesciences, Irvine, CA, USA) should be considered in patients when severe symptomatic FMR persists despite optimal guideline-determined medical therapy (GDMT) and revascularisation.
The description of the mitral regurgitation in the present case with a convergence area of the regurgitant flow involving the central part of the valve (A2-P2) is very untypical for an ischaemic MR, which is usually located at the coaptation level of A3-P3. This description suggests to the reader that a MitraClip procedure would probably be the only way to fix the problem. This might be true in the short term but one has to consider that functional mitral regurgitation is a heart failure problem, meaning that it is a ventricular and not a valvular problem. In this particular setting, the MitraClip fails to achieve any of the essential goals of reconstructive mitral valve surgery, although it may decrease the severity of FMR in the short term:
– it does not restore or preserve full leaflet mobility but immobilises the leaflets in their central part
– it does not restore a larger surface of coaptation but allows some coaptation in the middle of the leaflets and worsens coaptation in the non-central segments (A1-P1 and A3-P3)
– finally, it does not remodel the annulus for optimal orifice areas and long-term stability
– in addition, if later surgery becomes possible due to recompensation, preservation of the native mitral valve is uncertain due to the adhesions and damage to leaflets.
Cho et al examined long-term outcomes of non-heart transplant surgical approaches to advanced ischaemic cardiomyopathy (ICM), including left ventricular restoration (LVR) and mitral valve operation in 102 consecutive patients5. Thirty patients with greater than or equal to moderate mitral regurgitation (MR) underwent a mitral valve operation such as annuloplasty (n=23) or valve replacement (n=7). Patients were divided into four groups according to their Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) profiles: Profile 1-2 (the highest levels of clinical compromise; n=9), Profile 3-4 (n=40), Profile 5-6 (n=32) and Profile ≥7 (n=21). The four groups were compared: survival, major adverse cardiac and cerebrovascular events (MACCE), New York Heart Association (NYHA) status, LV volume and function. The overall eight-year survival including three hospital deaths (2.9%) was 64.3% without sudden death due to arrhythmia. A large majority of survivors (>90%) showed significant improvement in the mean NYHA status, from 2.9 to 1.4 (p<0.0001). The mean left ventricular end-systolic volume index (LVESVI) was significantly reduced from 104.1 to 61.4 ml/m2 (41% volume reduction) (p<0.0001). The improvements in NYHA status were significantly greater in patients with higher levels of clinical compromise (p<0.0001). This study confirms that MV repair is an excellent and durable option with long-term functional improvement. Interestingly, Acker and co-authors recently confirmed that the results after mitral valve repair and replacement for ischaemic mitral regurgitation are not significantly different6.
Nowadays, a large number of patients are at high risk or even contraindicated for surgery due to comorbidities. The MitraClip procedure may be a palliative option for some of these patients but the degree of reduction of mitral insufficiency is less with the MitraClip than with surgery7,8. Safety of the procedure is good in experienced centres. Most patients have functional improvement in the short and eventually mid term7. The procedure may be considered in patients who are at high risk for surgery and remain symptomatic despite optimal medical treatment. Both the technique and the equipment used in the procedure need to be improved, and a remodelling of the native valve annulus is a “must” for future interventional approaches.
In this case, I would use mechanical unloading and, exceptionally, a MitraClip.
Conflict of interest statement
The author has no conflicts of interest to declare.
How did I treat?
ACTUAL TREATMENT AND MANAGEMENT OF THE CASE
In consideration of the high surgical risk and the satisfying echocardiographic mitral valve characteristics, an interventional approach was indicated for the reduction of MR.
The procedure was performed through the right femoral vein under general anaesthesia and 2D and 3D TEE guidance. The MitraClip system (Abbott Vascular) consists of a tri-axial catheter system with an implantable clip9.
Elective mechanical circulatory support was carried out before the procedure by the Impella® system 2.5 L (Abiomed, Danvers, MA, USA) (Moving image 1). The device consists of a pigtail-like catheter within which is housed a micro-axial non-pulsatile pump capable of enhancing cardiac output by 2.5 L/min and requires a 12 Fr femoral sheath10. The Impella system was advanced through the left femoral artery into the middle LV cavity, according to the recommendations10. Right heart catheterisation was performed using a 6 Fr single-lumen, balloon-tipped Swan-Ganz catheter to obtain pulmonary capillary wedge pressure (PCWP), pulmonary artery pressure (PAP) and pulmonary artery oxygen saturation. Cardiac output (CO) was calculated by the Fick method.
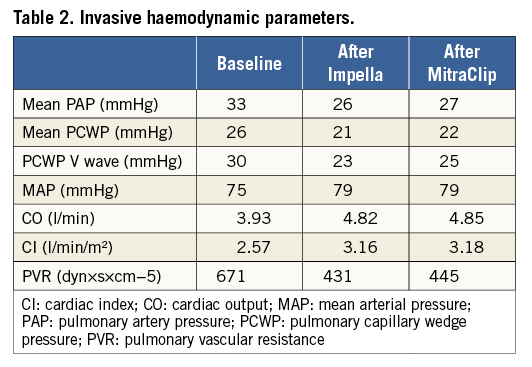
Following transseptal crossing, 70 IU/kg of unfractionated heparin was administered, maintaining an activated clotting time of >250 sec throughout the procedure. Then the 24 Fr guiding catheter was advanced into the left atrium (LA) and the delivery system was positioned in the LA (Figure 1, Moving image 2). Hence, the distal steerable part was manipulated in the atrium to obtain a perpendicular and central position with respect to the mitral coaptation line. Once the system had been properly aligned, the clip with opened arms was advanced into the LV, and under TEE guidance the arms grasped the leaflets. Finally, one clip was successfully implanted centrally between A2-P2 without complications (Figure 2). Intraprocedural TEE confirmed the reduction in MR from severe to mild (Figure 3) without significant gradient across the valve. Immediate haemodynamic changes during and after the procedures are shown in Table 2.
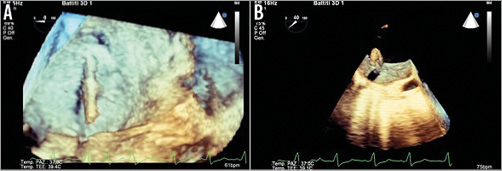
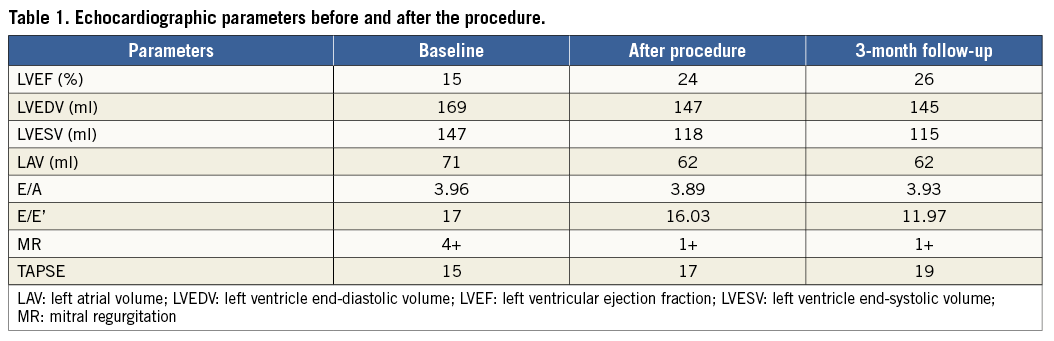
Figure 1. 3D TEE during clipping procedure. The steerable guide catheter (delivered with an echogenic tapered dilator) (A) and the MitraClip (B) advancing into the left atrium.
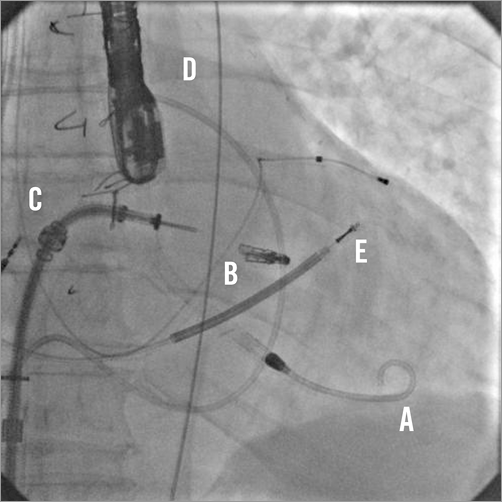
Figure 2. Fluoroscopic image depicting the deployment of the MitraClip. A) Impella 2.5 L; B) MitraClip; C) MitraClip delivery system; D) Swan-Ganz catheter; E) Implantable cardioverter-defibrillator catheter.
Figure 3. TEE in intercommissural and LVOT views showing 4+ MR before the procedure (A) and the residual 1+ MR after the MitraClip deployment (B).
The patient has been weaned from the Impella system support by decreasing gradually the performance level of the device, and the system was explanted at 24 hours following the intervention while a high dose of dobutamine was still being administered. Dobutamine infusion was therefore de-escalating, and stopped at 48 hours following the intervention. The patient was discharged on postoperative day five, in NYHA functional Class II. A pre-discharge TTE showed an improved LVEF (24%) and a reduction of LV systolic and diastolic diameters (Table 1).
At three-month follow-up the patient was asymptomatic with a NYHA functional Class I-II. TTE showed mild MR and improved LV function (LVEF 26%).
The percutaneous repair of the MR is a therapeutic option which serves as an alternative to conventional cardiac surgery in selected patients judged as inoperable by reason of a high or prohibitive surgical risk11,12. Initial clinical experience shows that percutaneous MV repair in patients with LV dysfunction can be performed safely with promising results in terms of efficacy1,9,13-16.
Nevertheless, due to the high-risk characteristics of the patients deemed suitable for the MitraClip procedure, the need for elective circulatory support should be taken into account. Indeed, although in the EVEREST II (Endovascular Valve Edge-to-Edge Repair) trial patients with severe LV systolic dysfunction (i.e., LVEF <20% and/or LVEDD >60 mm) were excluded13, several studies showed that percutaneous edge-to-edge repair may be safely performed in surgically high-risk patients with severely impaired LV systolic function1,15,16. In the Percutaneous Mitral Valve Repair in Cardiac Resynchronization Therapy (PERMIT-CARE) study, however, acute intraprocedural heart failure occurred in 7/51 (14%) patients, even with inotropic support either before or during the procedure1. Patients enrolled in that study had chronic moderate-to-severe or severe functional MR and severe LV systolic dysfunction (LVEF 27.1±8.7%). In the TRAnscatheter Mitral valve Interventions (TRAMI) registry16, 32.9% of patients had an LVEF <30%, and intraprocedural low cardiac output occurred in 9/1,164 patients (0.77%). Taramasso et al reported that, although there was a high inotropic support (defined as adrenaline dosage >0.5 γ/kg/min or association of two or more i.v. inotropic drugs), haemodynamic support by an intra-aortic balloon pump (IABP) was necessary in 14/109 (13%) patients and, in one patient, transient circulatory support with extracorporeal membrane oxygenation (ECMO)14. In the ACCESS-EU study15, on the contrary, no intraprocedural acute heart failure occurred, even though 62/562 (11%) patients had LVEF ≤20% and 4.9% were in cardiogenic shock at the time of the index procedure. Acute haemodynamic measurements obtained before and at least 10 minutes after MitraClip device deployment showed: 1) a 0.7 l/min mean cardiac output increment (from 3.7±1.5 l/min to 4.4±1.9 l/min), 2) a 3.5 mmHg decrease in the PCWP V-wave (from 23.0±10.8 mmHg to 19.5±9.1 mmHg), and 3) all other haemodynamic parameters stable post implant.
Circulatory support during MitraClip procedures has been provided by the intra-aortic balloon pump (IABP)14. However, despite ease of implantation, IABP provides limited (0.5 L/min) augmentation of cardiac output, which may be insufficient in cases of severely depressed LV function17. The Impella circulatory support catheter provides a higher rate of flow and, when compared to IABP, may allow for greater offloading of the left ventricle, which may help to decrease both systolic and diastolic wall stress. In the PROTECT II randomised trial, comparing Impella 2.5 L with IABP in patients undergoing high-risk PCI with severe LV systolic dysfunction, Impella was associated with superior haemodynamic support and a significant reduction in major adverse cardiac events at 90 days10.
To the best of our knowledge, this is the first case report describing a percutaneous mitral valve edge-to-edge repair with elective circulatory support carried out by the Impella system. The Impella system seems to be a safe and effective option allowing circulatory support in patients with severe MR and severe LV dysfunction undergoing MitraClip treatment.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Online data supplement
Moving image 1. Impella 2.5 L catheter crossing the aortic valve.
Moving image 2. MitraClip delivery system with the clip on the distal end, oriented to the left superior pulmonary vein.
Supplementary data
To read the full content of this article, please download the PDF.
Moving image 1. Impella 2.5 L catheter crossing the aortic valve.
Moving image 2. MitraClip delivery system with the clip on the distal end, oriented to the left superior pulmonary vein.

