
CASE SUMMARY
BACKGROUND: A 52-year-old lady with severe left ventricular dysfunction, severe mitral regurgitation and three-vessel disease, underwent coronary artery bypass graft on the left anterior descending artery and marginal branch, and mitral valve replacement with a biological prosthesis.
INVESTIGATION: Perioperative transoesophageal echocardiography.
DIAGNOSIS: Mitral prosthesis leaflet rupture probably caused by left ventricular vent removal.
MANAGEMENT: Transcatheter valve-in-valve procedure guided by transoesophageal echocardiography.
KEYWORDS: mitral left ventricular assist device, mitral prosthesis dysfunction, transatrial approach, transcatheter valve-in-valve
PRESENTATION OF THE CASE
A 52-year-old lady was admitted to our institution with a diagnosis of three-vessel coronary artery disease, left ventricular dysfunction and severe functional mitral regurgitation. A vitality test with thallium 201 scintigraphy showed viable tissue on the anterior and lateral walls. The left ventricle was hypertrophic, hypokinetic, with a 26% ejection fraction, and the echocardiographic parameters discouraged a mitral repair (the coaptation depth was 0.96 cm, while the tenting area was 2.4 cm²). Therefore, after preparation with 48 hours of levosimendan 0.05 µg·kg–1·min–1, she underwent a mitral valve replacement with the Mosaic® 29 mm bioprosthesis (Medtronic, Minneapolis, MN, USA) and coronary artery bypass graft on the left anterior descending artery and the obtuse marginal branch, with a total aortic cross-clamp time of 46 minutes. Unfortunately, despite maximal inotropic infusion and intra-aortic balloon pump, the patient experienced severe biventricular failure. Therefore, after a failed attempt at weaning the cardiopulmonary bypass (CPB), the patient was assisted with extracorporeal membrane oxygenator (ECMO) support. The aorta and right atrium were cannulated as is the routine approach for post-cardiotomy support. Then, given the severe left ventricular dysfunction, left ventricular venting was assured through the left atrium. The intra-aortic balloon pump was left in situ to allow the aortic valve to open.
The ECMO was beneficial and free from complications, lactates recovered, the renal function was preserved and no end-organ dysfunction was observed. Therefore, after six days, given the absence of left ventricle recovery, we decided to upgrade the circulatory support to an LVAD.
The patient was sent to the operating room, the previous sternotomy was reopened and the aorta and the right atrium were cannulated with new cannulae. Cardiopulmonary bypass was instituted while the ECMO was suspended and the previous cannulae were removed. Keeping the heart beating, the sewing ring of the inflow cannula was sutured onto the apex of the heart and a HeartWare HVAD® (HeartWare, Framingham, MA, USA) was placed. Subsequently, the outflow graft was sutured onto the lateral wall of the ascending aorta during partial aortic clamping. The procedure was straightforward without complications, and the CPB was weaned with satisfactory haemodynamics. Surprisingly, the intraoperative transoesophageal echocardiogram showed severe intraprosthetic mitral regurgitation, probably caused by leaflet rupture after the left ventricular vent removal (Figure 1). Despite LVAD revolutions-per-minute optimisation, the mitral regurgitation remained severe and the pulmonary pressure was high. The prosthetic regurgitation was considered detrimental for the right ventricular function; however, after a multidisciplinary discussion with the anaesthesiologist and the cardiologist, the traditional surgical prosthesis replacement was considered a high-risk procedure.
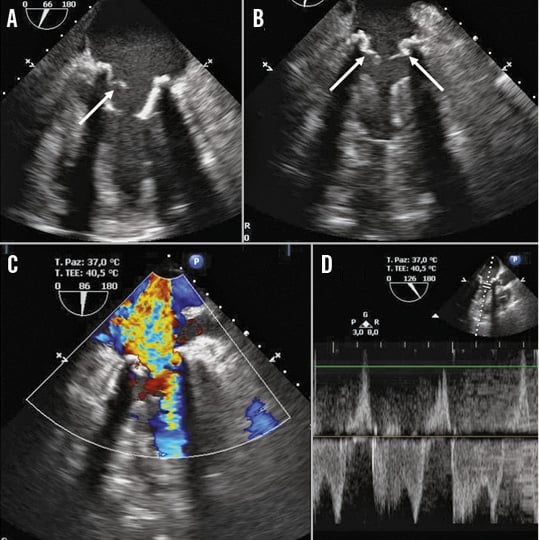
Figure 1. Transoesophageal echocardiographic views. Mitral prosthetic rupture (A, B, arrow). Severe regurgitation (C & D).
How would I treat?
THE INVITED EXPERTS’ OPINION

The authors present a most challenging case of a young female presenting with severe ischaemic left ventricular dysfunction and severe secondary mitral regurgitation. The patient underwent a short period of medical stabilisation/optimisation and subsequently had thallium-guided incomplete surgical myocardial revascularisation and mitral valve replacement with a 29 mm Medtronic Mosaic bioprosthesis. Postoperative cardiopulmonary bypass (CPB) weaning failed due to biventricular failure, and extracorporeal membrane oxygenation (ECMO) was required for stabilisation. Of note, left ventricular venting was performed via the left atrium. In the absence of left ventricular recovery, a redo sternotomy was performed to implant a HeartWare HVAD. Weaning of the CBP was successful, but mitral bioprosthetic leaflet rupture causing severe mitral regurgitation and cardiac embarrassment was noticed following CBP weaning.
The authors’ management of this critically ill patient is to be commended, and they have demonstrated significant skill and judicious use of medical technology. We would, however, question the incomplete myocardial revascularisation based on a thallium scan, the added benefit of the intra-aortic balloon pump after establishing venous-arterial ECMO, and the decision to proceed to LVAD so quickly, especially given the excellent response to ECMO. The rupture of the mitral prosthesis is likely to have occurred due to valve trauma upon withdrawal of the left atrial vent or interaction of the left atrial vent with the proprietary coring tool used to facilitate the placement of the left ventricular sewing ring of the HeartWare HVAD.
Now faced with the mitral leaflet rupture, two immediate treatment options exist: 1) re-establish CPB for a third time and surgically replace the mitral valve, or 2) perform a transcatheter aortic valve implantation within the mitral prosthesis. This latter option could be performed either following de-airing the heart under direct visualisation, or on the beating heart. A transapical procedure is not possible, given the position of the LVAD inflow cannula; therefore, a transatrial implant would be more appropriate. A 29 mm Mosaic has an internal diameter of 26 mm, and a “true” internal diameter of 24 mm. Therefore, either a 26 or a 29 mm Edwards SAPIEN XT (Edwards Lifesciences, Irvine, CA, USA) could be implanted under fluoroscopic and transoesophageal echocardiographic guidance.
Conflict of interest statement
D. Mylotte is a consultant for Medtronic and MicroPort.. N. Piazza is a consultant for Medtronic and MicroPort; T. Modine is a consultant for Medtronic and MicroPort.
How would I treat
THE INVITED EXPERTS’ OPINION

The authors need to treat a severely regurgitant Mosaic 29 mm mitral bioprosthesis implanted a few days previously, while still in the operating room and upgrading an ECMO to a HeartWare HVAD in a young patient with severe end-stage ischaemic heart failure and recent mitral valve replacement. A possible reason for severe intraprosthetic regurgitation at this stage is leaflet damage induced by the removal of the LV venting cannula implanted via a left atriotomy and used to drain blood in the venous ECMO cannula, as shown by operative transoesophageal echocardiography.
In a very similar case, Stamm et al observed leaflet thrombosis induced by prolonged immobilisation of the bioprosthetic leaflet by the cannula itself as another potential cause of bioprosthesis dysfunction1. In this case report, the authors brilliantly solved the issue through surgical access to the left atrium, excision of all three bioprosthetic leaflets and implantation of an Edwards SAPIEN 29 mm TAVI device into the frame of the bioprosthesis under direct vision. The procedure was successfully performed without additional aortic cross-clamping on the beating heart.
In the present case, since the authors became aware of the problem after cardiopulmonary bypass (CPB) weaning, a similar solution with a transcatheter mitral valve-in-valve would appear intriguing, as it could avoid additional aortic cross-clamping and potentially restore mitral valve function quickly and effectively. Surgical leaflet excision could also possibly be avoided, as it is never performed during standard transcatheter mitral valve-in-valve procedures. Since CPB has been removed, surgical access to the left atrial cavity cannot be performed. An Edwards SAPIEN XT 26 or 29 mm valve could be implanted under fluoroscopic (if available) or transoesophageal echo guidance inside the Mosaic 29 after accessing the left atrium with a dedicated TAVI sheath and crossing the MV prosthesis leaving a guidewire in the left ventricle. Alternatively, the authors could reinstitute CPB and perform a mitral valve-in-valve under beating heart and direct visualisation as described by Stamm et al. However, this procedure has the potential complications related to CPB reinsertion. Furthermore, the off-pump valve-in-valve procedure avoids prolonged extracorporeal circulation and eventual further aortic cross-clamp, which in this setting could be particularly detrimental to right ventricular function, thus compromising a successful postoperative LVAD management.
Mitral valve-in-valve is a well-documented procedure, commonly used to treat structural valve deterioration in MV prostheses2. The only potential concern for this specific patient would be the long-term results in terms of durability given her young age. A potential justification for its adoption is the fact that risk-benefit evaluation urges a resolution of the acute problem, and long-term results appear less important in this acute heart failure setting.
Conflict of interest statement
The authors have no conflicts of interest to declare.
How did I treat?
ACTUAL TREATMENT AND MANAGEMENT OF THE CASE
The traditional surgical approach with aortic clamping and prosthetic replacement was excluded given the borderline right ventricular function. Therefore, the patient was considered for a transcatheter valve-in-valve procedure with an Edwards SAPIEN 3 prosthesis (Edwards Lifesciences).
In addition, there were a few more issues concerning the procedure, the first being the surgical approach. The transapical approach was excluded in order to avoid excessive mobilisation of a heart with a mitral prosthesis. We therefore chose to go through the left atrium using a transapical kit. Second, the guide of the deployment kit, passed through the ventricle and close to the inflow cannula of the LVAD, could harm the working centrifugal pump, so we decided to restart the cardiopulmonary bypass and stop the LVAD under full heparinisation for the duration of the procedure. Third, the LVAD implantation procedure was not performed in a hybrid operating room, so fluoroscopy and the C-arm were not available. Therefore, the procedure was achieved under transoesophageal and epicardial echocardiography.
Surgical technique
CPB was reinstituted, the LVAD was stopped and a 3.0 mm polypropylene purse-string suture was made on the left atrium, just medially to the previous surgical suture. An 18 Fr Certitude sheath (Edwards Lifesciences) was passed through the purse string and the guidewire was left in place. Since the transoesophageal echocardiogram did not allow a perfect coaxial alignment of the valve, an epicardial probe was used, obtaining a good window (Figure 2). Eventually, a 26 mm Edwards SAPIEN 3 prosthetic valve was deployed.
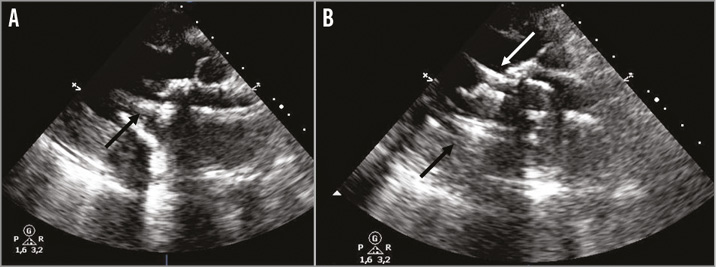
Figure 2. Epicardial echocardiographic views. Alignment of the delivery system (A, black arrow). The correct deployment of the valve-in-valve (B, white arrow).
The final result was excellent, and no residual prosthetic regurgitation was detected (Figure 3). The CPB was finally weaned and the LVAD restarted at 2,500 rpm. The mean pulmonary pressure was 25 mmHg, and the wedge pressure was 19 mmHg, with a mean arterial blood pressure of 75 mmHg.
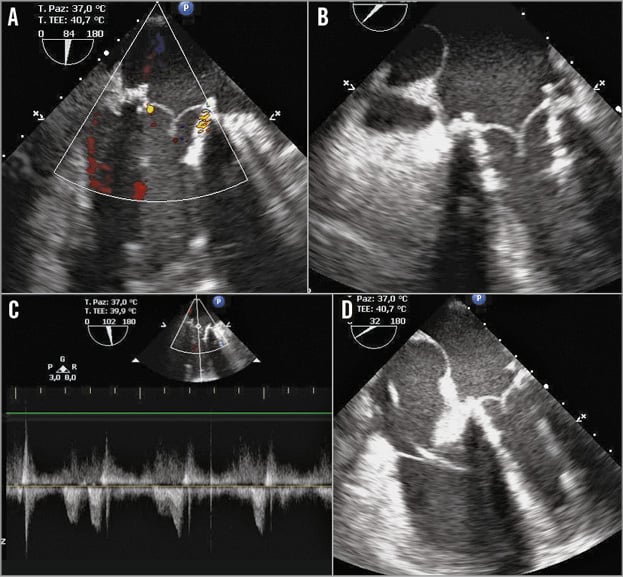
Figure 3. Transoesophageal echocardiographic views. The valve-in-valve position (B & D), without regurgitation or paravalvular leaks (A & C).
Unfortunately, the patient died a few days later after a severe septic shock with right ventricular failure.
Successful mitral transcatheter valve-in-valve implantation (TVIV) procedures have been reported previously and clinical feasibility has been confirmed3-8. However, these reports described mainly transapical procedures separately or simultaneously with transapical aortic valve implantation9. The transatrial approach has been described previously10. However, to our knowledge there are no reports of mitral TVIV with the transatrial approach in patients on long-term mechanical support.
The approach we described can be a useful bail-out opportunity in case of difficult or impossible access to the apex of the heart. Nevertheless, it needs the strict multidisciplinary collaboration of the Heart Team members. Ideally, for an elective procedure, it should be performed in a hybrid room, to have both fluoroscopy and transoesophageal echocardiographic support. Nevertheless, epicardial echocardiography can be an option in case of challenging transoesophageal alignment when a hybrid room is unavailable.
Conflict of interest statement
The authors have no conflicts of interest to declare.

