
Abstract
We report the first-in-man implantation of the Mitra-Spacer. The device was implanted transapically. FMR was reduced to moderate. At two months, while in NYHA Class II, LVEF had improved, but FMR increased and 2 mL was added, reducing FMR to mild. Despite anticoagulation, thrombi developed around the device and the valve was replaced at eight months. The Mitra-Spacer successfully bridged this patient to surgery after LVEF had recovered.
Introduction
Mitral valve (MV) surgery is the standard treatment in MV disease1. However, many patients are at high risk for surgery. Devices for transcatheter MV repair either reduce annular dimensions or alter leaflet mobility. Nevertheless, procedures have limited efficacy due to recurrent functional mitral regurgitation (FMR)2,3.
Transcatheter MV treatment remains challenging: the MV annulus is large, not circular, three-dimensional and dynamic in geometry. Possible interaction with chordae or the left ventricular (LV) outflow tract can be fatal and device fixation is challenging. Paravalvular leakage is less well tolerated and device thrombosis is a risk.
Therefore, new devices were developed to improve leaflet coaptation, without changing MV anatomy. These “intra-valvular spacers” are fixed to the LV apex and placed into the regurgitant MV orifice. This concept has been tested in animals4 and temporarily in humans5. We report the first-in-human experience of the Mitra-Spacer™ (Cardiosolutions, Inc., West Bridgewater, MA, USA).
Methods
PATIENT
A 58-year-old male had severe FMR and heart failure (HF) (LVEF: 28%) six weeks after a myocardial infarction (Figure 1, Moving image 1, Moving image 2). After PCI, he remained dialysis-dependent, in NYHA Class III and hypotensive, which precluded HF medication.
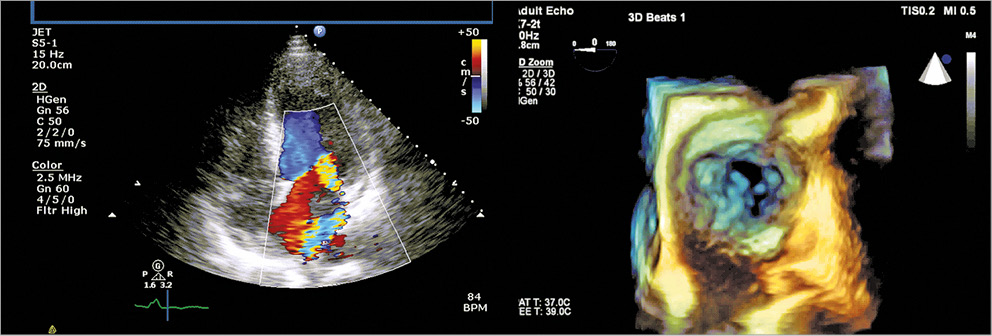
Figure 1. Transthoracic and 3D echo images showing severe functional MR.
The patient was considered too high risk for surgery. Various treatment options (MitraClip, cardiac resynchronisation therapy, and transplant) were turned down. Regulatory approval was obtained for compassionate use of the Mitra-Spacer.
DEVICE
This device reduces FMR by occupying space in the MV orifice, increasing native leaflet coaptation (Figure 2). The Mitra-Spacer is a fluid-filled balloon, connected to the HeartPad® (B. Braun, Melsungen, Germany), an apical anchor. The Mitra-Spacer is available in one size (compressed flat balloon width: 25 mm). The implantation is transapical (18 Fr). The balloon is connected to a subcutaneous port for later adjustment by fluid retrieval or filling. The material is Elast-Eon™ (AorTech, Weybridge, Surrey, United Kingdom), a non-thrombotic polymer. It has been tested for biocompatability and thrombogenicity beyond 100 million cycles and has been tested in 13 animals for chronic use.
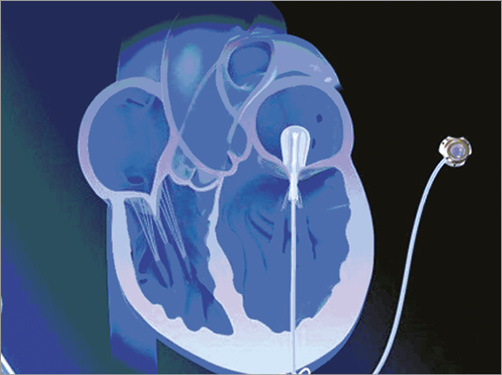
Figure 2. Schematic illustration of the concept.
PROCEDURE
Guidance was fluoroscopic and echocardiographic. The balloon was advanced until approximately halfway into the left atrium (Figure 3) and locked using the HeartPad. Stepwise, it was filled with fluid, with FMR being graded based on the colour-flow jet width, dispersed circularly around the balloon (Moving image 3). Flow reversal in the pulmonary veins was also measured. The best FMR reduction was observed at a filling of 7.5 ml of fluid (Figure 4), while cardiac output increased (Figure 5). The port was implanted into the subclavian fossa (Moving image 4).
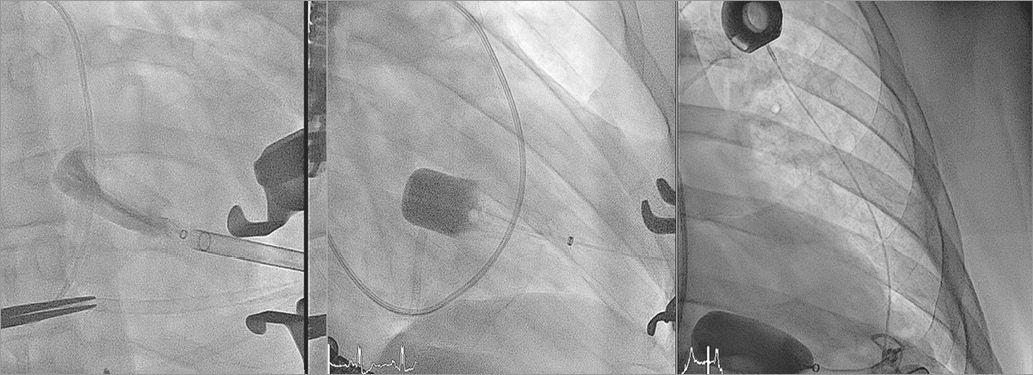
Figure 3. Fluoroscopy images of procedural steps.
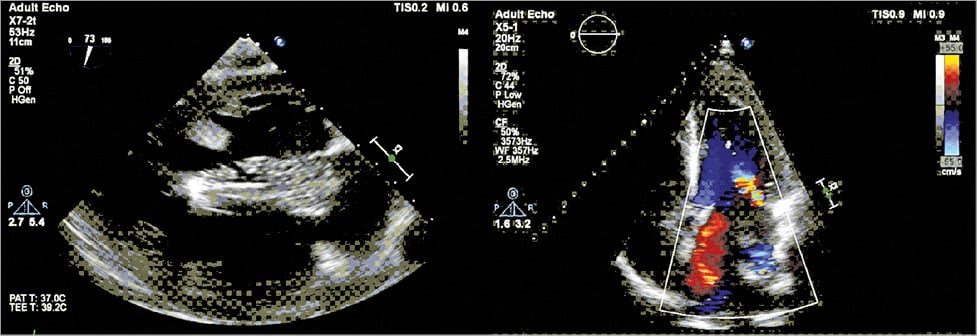
Figure 4. Final position and assessment of residual MR at the end of the procedure.
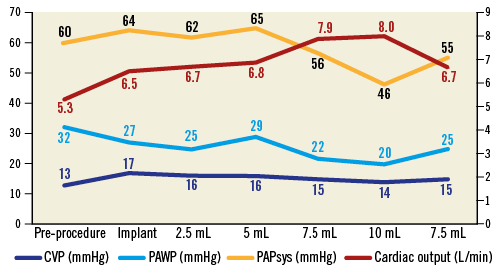
Figure 5. Haemodynamic measurements during implantation. CVP: central venous pressure; PAPsys: systolic pulmonary artery pressure; PAWP: pulmonary artery wedge pressure
Results
FMR was moderate post procedure. The patient had an uneventful course, was no longer dialysis-dependent and amenable to HF medication. Creatinine decreased from 285 mmol/L to 185 mmol/L. There were no adverse events and he was discharged on day five on warfarin and dual antiplatelet therapy. During a period of 30 days, there was one re-admission for cardiac decompensation due to fast atrial flutter. At six weeks, he was in NYHA Class II.
At seven weeks he was admitted with a transitory ischaemic attack. Echocardiography showed a thrombus around the shaft of the balloon. The INR target was increased to 3.5 and the thrombus resolved. LVEF improved (35%) but FMR increased, to moderate-severe.
At four months, the patient remained in NYHA Class I-II with improved LVEF but unchanged left ventricular end-diastolic diameter (LVEDD) (45%, 69 mm), resulting in increased FMR. The Mitra-Spacer was filled with a further 2.5 ml of fluid under echocardiographic guidance (final volume: 10 ml), resulting in mild FMR (Moving image 5).
At eight months, a thrombus again formed around the balloon (Moving image 6). Since the patient had improved LVEF and clinical status, he was now suitable for surgery. The Mitra-Spacer was removed; the leaflets were completely intact. However, MV repair failed due to a severely restrictive posterior leaflet and dilated ring and therefore the MV was replaced. At 18 months, the patient has completely recovered and is in NYHA Class I.
Discussion
MV surgery restores MV competency. However, in patients with FMR and poor LV function, a suddenly competent MV increases afterload, resulting in additional LV stress. Thus, the incidence of acute perioperative left HF is increased. Interventional treatment options are feasible; however, implantation is technically challenging with questionable long-term results.
In contrast, Mitra-Spacer implantation is not an attempt to treat the complex MV directly, but to reduce FMR and re-establish a connection between the MV and the LV. Implantation is simple and the port allows later adjustment of balloon size, reducing FMR stepwise and giving the LV time to improve with a reduced incidence of acute HF and recurrent FMR. The opportunity for dynamic adjustment of the balloon is a unique feature of the Mitra-Spacer, not offered by any other MV technology. As there is no interaction with the MV or annulus, there is no risk of LV outflow tract obstruction. While we achieved satisfactory results, the device also showed increased thrombogenicity despite oral anticoagulation. Therefore, it is contraindicated in patients unable to take oral anticoagulation. Currently, adjustments on the device are being made to reduce thrombogenicity.
Limitations
This is an initial experience. Only clinical trials can show whether this technique is beneficial in comparison to the existing portfolio of percutaneous MV devices.
Conclusions
This first-in-man experience using the Mitra-Spacer shows its feasibility in patients with LV dysfunction and FMR. While the Mitra-Spacer can decrease FMR, it may also improve LV function and quality of life. Although the device does not leave the option of treatment with other percutaneous devices, its minimal interference with MV anatomy preserves the option of later MV repair. The concept should be developed for endovascular delivery routes. Also, the device should be further adjusted to reduce thrombogenicity.
| Impact on daily practice Patients with FMR are often unsuitable for surgery and interventional treatment options lack efficacy. The Mitra-Spacer offers an option for dynamic transcatheter mitral valve repair. Patients with FMR not suitable for surgery could benefit from this technique. |
Funding
The research posts of M. Silaschi, O. Aldalati and M. Eskandari are funded through the King’s College Hospital Charity, London, United Kingdom.
Conflict of interest statement
M. Silaschi has received travel compensation from Cardiosolutions, Inc. O. Wendler is a consultant for Cardiosolutions, Inc. T. Piemonte is CMO at Cardiosolutions, Inc. The other authors have no conflicts of interest to declare.
Supplementary data
Moving image 1. Preoperative 3D echo of the MV.
Moving image 2. Echo showing baseline FMR.
Moving image 3. Intraoperative echo of the Mitra-Spacer in place.
Moving image 4. Fluoroscopy of the Mitra-Spacer and port in place.
Moving image 5. Echo showing FMR after adjustment of the filling volume.
Moving image 6. Echo showing thrombus around the shaft of the balloon.
Supplementary data
To read the full content of this article, please download the PDF.
Preoperative 3D echo of the MV.
Echo showing baseline FMR.
Intraoperative echo of the Mitra-Spacer in place.
Fluoroscopy of the Mitra-Spacer and port in place.
Echo showing FMR after adjustment of the filling volume.
Echo showing thrombus around the shaft of the balloon.

