Abstract
Aims: Percutaneous mitral valve repair (pMVR) is a new therapeutic option for mitral valve regurgitation. Positive preliminary results in non-randomised studies have been published supporting the use of the MitraClip system in patients with secondary mitral regurgitation (MR) and poor left ventricular (LV) function contraindicated to surgery. The aim of the MITRA-FR study is to provide a higher level of evidence for the efficacy of the MitraClip device in this setting.
Methods and results: The MITRA-FR study is a national, multicentre, investigator-initiated, open-label, randomised trial to evaluate the benefits and safety of pMVR using the MitraClip system plus optimal medical therapy (OMT) compared with OMT alone (control) in patients with severe symptomatic secondary MR contraindicated to surgical repair. The trial aims to enrol 144 MitraClip-treated subjects and 144 control (OMT alone) patients. The primary endpoint is a composite of all-cause mortality and unplanned hospitalisations for heart failure at 12 months after randomisation.
Conclusions: MITRA-FR is a randomised controlled national trial designed to evaluate the performance of pMVR in comparison to OMT in patients with severe symptomatic secondary MR contraindicated to cardiac surgery.
Abbreviations
ACC: American College of Cardiology
ACEI: angiotensin-converting enzyme inhibitor
AHA: American Heart Association
BNP: brain natriuretic peptide
CABG: coronary artery bypass grafting
CE: Conformité Européenne
CRT: cardiac resynchronisation therapy
DSMB: data safety monitoring board
EACTS: European Association of Cardio-Thoracic Surgery
eCRF: electronic case report form
ESC: European Society of Cardiology
FDA: Food and Drug Administration
HF: heart failure
LV: left ventricular
LVEF: left ventricular ejection fraction
MR: mitral regurgitation
MV: mitral valve
MVR: mitral valve repair
NYHA: New York Heart Association
OMT: optimal medical therapy
pMVR: percutaneous mitral valve repair
TAVI: transcatheter aortic valve implantation
TOE: transoesophageal echocardiographic
TTE: transthoracic echocardiographic
Introduction
Mitral regurgitation (MR) is the second most frequent valve disease requiring surgery in European countries1. MR is separated into primary MR (organic lesion of the valve) and secondary MR (functional MR with dilated ventricles mainly in ischaemic or idiopathic cardiomyopathy). Primary MR remains an indication for surgical mitral valve (MV) repair as confirmed by the European Society of Cardiology (ESC)/European Association of Cardio-Thoracic Surgery (EACTS) and American Heart Association (AHA)/American College of Cardiology (ACC) guidelines2,3.
Conversely, and despite a worse prognosis, surgical indications for secondary MR are still debated due to controversial results and no demonstrated benefit on clinical outcomes. The potential benefit of secondary MR surgical correction is counterbalanced by an increased postoperative mortality and a lower five-year survival rate4. These results may be explained by risk factors such as left ventricular dysfunction, advanced age or the presence of comorbidities5. Moreover, the recurrence of MR after MV repair is high. The only recommendation class I or IIa surgical indications for severe secondary MR correspond to the association with coronary artery bypass grafting (CABG), while it is only a recommendation class IIb in patients remaining symptomatic despite optimal medical therapy (OMT), including cardiac resynchronisation therapy (CRT) when there is no option for CABG or in patients remaining symptomatic despite OMT2,3. On the other hand, the clinical outcome of unoperated, medically managed patients with secondary MR is poor with 12-month and five-year mortality rates of 20% and 50%, respectively6. This emphasises the need for low-risk procedures to improve this population’s survival.
As for aortic valve disease and the emergence over the last decade of transcatheter aortic valve implantation (TAVI), innovative percutaneous approaches have been developed to enable MV repair (MVR) in patients with severe MR7,8. Percutaneous MVR (pMVR) using the MitraClip® system (Abbott Vascular, Santa Clara, CA, USA) is the most advanced of all with more than 15,000 procedures performed worldwide. To date, there are no data from head-to-head comparisons to demonstrate the effectiveness of pMVR in addition to OMT. We describe the design of a randomised controlled trial to assess the clinical efficacy of pMVR plus OMT in comparison to OMT alone in patients with severe secondary MR contraindicated to surgery.
Methods
STUDY DESIGN AND OBJECTIVES
MITRA-FR is a prospective, multicentre, randomised, open-label, controlled study of the MitraClip system for the treatment of severe secondary MR.
The study is being performed according to the principles of the Declaration of Helsinki, and approval has been obtained from ethics committees in France. The study has also received authorisation from the French National Agency for Medicines and Health Products Safety on 23 May 2013 (registration number: 2013-A00464-41). The trial is registered at www.clinicaltrials.gov (identifier: NCT01920698).
All participants give written informed consent prior to enrolment in the study.
The primary objective is to demonstrate that pMVR reduces the occurrence of all-cause mortality or unplanned hospitalisations for heart failure (HF) at 12 months in comparison to OMT alone.
The secondary objectives are to compare the two strategies in terms of morbidity, mortality, safety, quality of life, echocardiographic evaluation, biomarkers, functional evaluation, and cost-effectiveness.
DEVICE AND PROCEDURES
The investigational device is the MitraClip system (Abbott Vascular) designed to perform pMVR of the regurgitant MV. This medical device obtained the Conformité Européenne (CE) certificate in March 2008 and Food and Drug Administration (FDA) approval in October 2013 (with limitation to severe primary MR).
The MitraClip system consists of a clip delivery system and a steerable guide catheter. The implantation procedure starts with a venous femoral access and a transseptal puncture under fluoroscopy, plus two- and three-dimensional transoesophageal echocardiography. The clip delivery system is introduced into the left atrium. The steerable guide is then introduced and the clip is positioned in the middle of the regurgitant jet where the two leaflets are grasped and clipped. After assessment of the decrease in the degree of regurgitation, the decision to deliver or reposition the clip is taken. Additional clip implantation is possible in case of unsatisfactory control of regurgitation.
STUDY POPULATION
Eligible patients include those with severe secondary MR who are contraindicated for heart surgery by a multidisciplinary Heart Team. All inclusion and exclusion criteria are presented in Table 1 and Table 2.


SUBJECT SCREENING, ENROLMENT, AND RANDOMISATION
All patients are screened for study eligibility by the principal investigator at each site. Each patient’s case is presented at a multidisciplinary meeting of a Heart Team according to the recent guidelines2. The Heart Team includes at least a surgeon, an interventional cardiologist, and a general cardiologist. They confirm the contraindication to valve surgery and the persistence of HF symptoms despite optimal medical management. This contraindication takes into account the risks and benefits of an MV surgical intervention according to the patient’s status and comorbidities, as well as the current recommendations in this type of clinical situation. This contraindication decision is based upon a consensus among all members of the Heart Team. According to the latest ESC guidelines10, optimal therapy combines pharmacological and non-pharmacological options. The first consists of the use of an angiotensin-converting enzyme inhibitor (ACEI) (or angiotensin receptor blocker in patients intolerant of an ACEI), a beta-blocker, and aldosterone receptor antagonist. CRT with or without implantable cardiac defibrillator is considered as part of the optimal therapeutic management in patients presenting with eligible indications according to updated guidelines. In all centres, each case is discussed within the Heart Team and the indication for CRT as well as atrial fibrillation ablation or cardioversion and coronary revascularisation is assessed thoroughly. The opinion of other specialists can be required, at the discretion of each centre’s Heart Team.
Based on both transthoracic echocardiographic (TTE) and transoesophageal echocardiographic (TOE) examinations, the vascular access and favourable anatomical conditions are checked locally. Then, the patient is considered eligible for pMVR.
The echocardiographic data are sent by centres to a centralised echocardiography core laboratory for review by independent experts. The core laboratory validates the MR echocardiographic selection criteria for each patient before final inclusion.
The patient’s characteristics and the clinical and echocardiographic data at the inclusion are collected using an electronic case report form (eCRF).
The randomisation is performed after the inclusion of the patient using a secure and dedicated web server. Randomisation is centralised and stratified by participating centre. Patients are randomised in a 1:1 ratio between the two arms.
If the patient is randomised to the MitraClip system arm, the procedure is scheduled within 21 days after randomisation. All procedures are performed with proctoring from Abbott Vascular (i.e., the presence of technician specialists during the procedures). One or two additional MitraClips can be used during the procedure when needed. In case of an unsatisfactory result after the procedure has been completed, no additional MitraClip procedure is allowed at any time during the two years of follow-up.
Post-implantation thromboprophylaxis includes acetylsalicylic acid (75 mg per day) indefinitely and clopidogrel (75 mg per day) for three months. In case of concomitant atrial fibrillation or another condition necessitating anticoagulant therapy, the anticoagulation regimen includes a vitamin K antagonist indefinitely plus acetylsalicylic acid (75 mg per day) for three months.
No crossover is allowed between the two arms during the two years of follow-up.
PATIENT FOLLOW-UP, DATA COLLECTION
The investigation schedule is depicted in Figure 1. The 30-day, six-month, 12-month and 24-month post-randomisation, follow-up data are collected during clinical visits, or by telephone interview for the 30-day data in the control non-interventional trial arm.
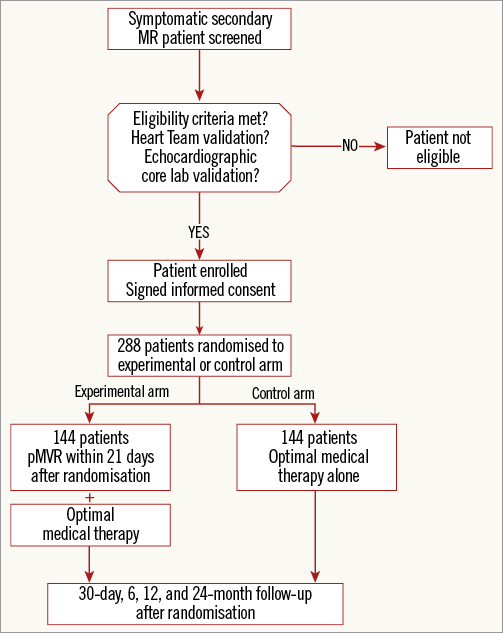
Figure 1. MITRA-FR investigation schedule.
PRIMARY AND SECONDARY ENDPOINTS
The primary endpoint is a composite outcome of all-cause death and unplanned hospitalisations for HF, 12 months after randomisation.
The secondary endpoints are all-cause death, cardiovascular death, major cardiovascular event-free survival, and frequency of serious adverse events at 30 days, six months, 12 months, and 24 months after randomisation.
The other secondary outcome measures are the change in quality of life score (European Quality of Life-5 Dimensions instrument), the change in echocardiographic evaluation (pulmonary pressure, left ventricular diameter, grade of MR, left ventricular ejection fraction [LVEF]), measures being performed by the echocardiography core laboratory, the change in biomarkers (brain natriuretic peptide [BNP] levels, creatinine), the change in functional evaluation (New York Heart Association [NYHA] class, six-minute walk test), and the estimation of the incremental cost-effectiveness ratio expressed as a cost per major cardiac event avoided (perspective: French sickness fund, time horizon: 12 months)
ADJUDICATION OF ENDPOINTS
All endpoints are adjudicated by an independent endpoint validation committee.
It will be constituted by at least three physicians experienced in clinical research, including a cardiac surgeon, an interventional cardiologist, and a medical cardiologist. Only the adjudicated endpoints will be used for all statistical analyses.
DATA SAFETY MONITORING BOARD
An independent data safety monitoring board (DSMB) is set up specifically to monitor safety data throughout the study duration, and to determine if it is appropriate, from both the scientific and the ethical standpoint, to continue the study as planned. The DSMB consists of three members: a cardiac surgeon, a cardiologist, and a statistician. It will evaluate the types and proportions of serious adverse events reported at 30 days post randomisation, after inclusion of 33% and 66% of the study population.
Statistical considerations
SAMPLE SIZE CALCULATION
The percentage of all-cause mortality or unplanned hospitalisations for HF at 12 months was assumed to be 50% in patients with severe secondary MR benefiting from OMT alone. This is consistent with the results describing the clinical outcomes of a US cohort of unoperated patients with severe MR6 (all-cause mortality at 12 months: 20%; proportion of patients with congestive HF: 41%). For patients in the interventional arm, the proportion of composite events was estimated to be 33% based on the preliminary results of the EVEREST II High Risk Study11 (all-cause mortality at 12 months: 22.8%; proportion of patients with congestive HF: 19.8%). Based on these assumptions, 131 patients are needed per group to demonstrate a difference between groups using a chi-squared test (power of 80%, alpha risk of 5%), representing 262 patients overall12. To take into account lost to follow-up patients (10%), a total of 288 patients are being recruited.
STATISTICAL ANALYSIS
The statistical analysis will be performed by an independent statistical department. The primary analysis will be carried out on the intention-to-treat population, defined as all randomised patients, whether they received the study treatment or not. A per-protocol analysis will also be performed to evaluate pMVR under optimal conditions. It is planned to account for missing outcome data by multiple imputation and/or sensitivity analyses.
A descriptive analysis of included patients will be performed on all recorded parameters. The hypothesis of normal distribution of quantitative variables will be tested using the Kolmogorov-Smirnov test and graphically confirmed with a histogram. If necessary, the variable will be converted to log and/or specifically analysed after exclusion of outliers. Patients’ characteristics at the time of randomisation in the two arms will be compared using the chi-squared test or Fisher’s exact test when the conditions of application of the chi-squared test are not met for categorical variables. Quantitative variables will be compared between groups using the Student’s t-test after verification of equality of variances when data are normally distributed, and with Wilcoxon’s non-parametric test when the hypothesis of normality of distribution is not verified.
The proportion of all-cause deaths or unplanned hospitalisations for HF 12 months after randomisation will be estimated with its 95% confidence interval. The proportions will be compared between the two arms using the chi-squared test. For secondary outcome measures, categorical variables will be compared using the chi-squared test or Fisher’s exact test, as detailed above. Quantitative variables will be compared using the Student’s t-test or the Mann-Whitney U test, as appropriate.
Survival will be described and estimated with the Kaplan-Meier method, and compared using the log-rank test. A Cox proportional hazard model will be fitted, and the proportional hazard hypothesis will be checked with Schoenfeld residuals. The statistical tests are bilateral and the level of significance has been set at 5%. The information presented above forms the basis for the statistical analysis plan. Any other analysis will be pre-specified in the statistical analysis plan before database lock. No subgroup analysis is planned. Two interim safety analyses are planned after inclusion of 1/3 and 2/3 of the total number of patients. No interim analysis for efficacy based on the primary endpoint is planned. Analyses will be performed using SAS 9.3 or a later version (SAS Institute, Cary, NC, USA).
Trial organisation
The MITRA-FR study is promoted by the Clinical and Innovation Research Department of Lyon University Hospitals (France), which is responsible for study monitoring. Trial coordination is performed by the Centre of Clinical Investigation of Lyon University Hospitals, and statistical analyses are under the responsibility of Lyon University Hospitals Biostatistics Department. The trial structure also includes the following groups: the Steering Committee, the Echocardiography Core Laboratory (located in the Xavier Bichat Hospital, Paris, France), the Adjudication Committee, and the DSMB.
The participating centres of the MITRA.FR trial have been selected based on the following criteria: 1) expertise in valvular heart disease and its management through a TAVI programme; 2) presence of a structured Heart Team already used to performing accurate and multidisciplinary patient selection. Furthermore, centres have to justify having experience of at least five MitraClip procedures before entering the study.
Current status of the trial
The first patient was enrolled in December 2013. To date, the MITRA-FR trial has randomised 80 patients from 19 participating centres. Patients’ mean age was 71±12 years, 70% were male, 54% had a history of ischaemic heart disease and mean left ventricular ejection fraction was 29.9%.
Discussion
Mitral regurgitation is associated with an increased risk of mortality and morbidity, and an altered quality of life; however, prospective clinical trial data regarding management of patients with severe symptomatic secondary MR are lacking. Many patients are not considered appropriate candidates for surgery because of altered LVEF, age and/or presence of multiple comorbidities. Medical therapy improves HF symptoms, morbidity, mortality, and left ventricular (LV) remodelling in systolic dysfunction, but its impact on MR is poor. The presence of moderate to severe MR is a strong independent predictor of death in systolic HF patients13. Moreover, a relationship between increasing MR severity and greater mortality has been observed in HF patients with systolic dysfunction14. Hence, alternative strategies and therapies are needed in these patients when surgery is contraindicated.
pMVR using the MitraClip system is a new option for patients with severe secondary MR. The MitraClip system was initially developed through the EVEREST programme, mainly represented by a randomised trial comparing pMVR to surgery in patients with severe MR (73% primary/27% secondary)15,16. Several registries have confirmed the procedure’s feasibility as well as its low procedural risk, and have shown promising results in terms of improvement of MR grade, functional outcome, and quality of life17-22. These results are based on a limited number of patients with severe secondary MR and at high surgical risk, followed up for a short- to mid-term duration, and are hampered by the influence of potential confounding factors. Within the commercial practice of the MitraClip system, there has been a shift in the indications for pMVR, secondary MR representing up to 65% of aetiologies in the most recent updates23. With regard to its potential benefit, the ESC guidelines have considered pMVR as a potential option in “patients with symptomatic severe secondary MR despite optimal medical therapy who fulfil the echo criteria of eligibility, are judged inoperable or at high surgical risk by a Heart Team”2. Furthermore, the FDA has approved pMVR with the MitraClip system as an option for patients with severe primary MR and at high surgical risk due to existing comorbidities. The indication of pMVR in this population is the only one considered in the latest AHA/ACC guidelines3.
While the MitraClip system is increasingly used in Europe, the level of evidence supporting pMVR in secondary MR patients contraindicated to surgery is low (recommendation class IIb, level of evidence C). The impact of pMVR on mortality and morbidity in patients with optimal medical therapy has to be assessed with prospective, comparative, randomised studies such as the MITRA-FR and the COAPT trials. These studies, as well as the RESHAPE-HF trial which has just been terminated, have large similarities in terms of inclusion criteria and primary outcome measures (Table 3). However, they differ in several respects. First, the upper value of the LVEF range of the COAPT trial’s inclusion criteria is substantially higher than in the other two studies. Second, the sample sizes vary from a factor of almost one to three between studies which can be explained by different assumptions on primary effectiveness endpoints. Finally, the studies were designed under different circumstances. Despite the MitraClip system being commercially available (CE mark in 2008), access to it varies greatly between European countries.
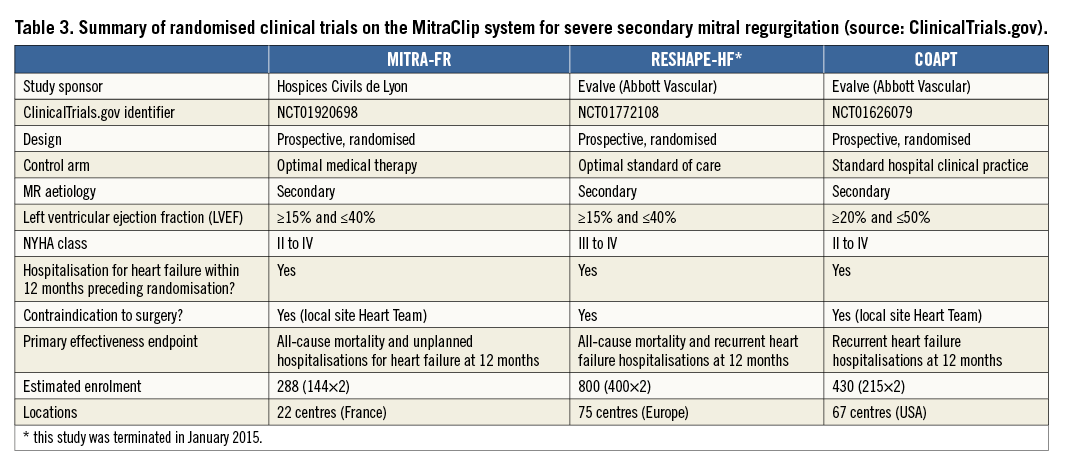
In France, the MitraClip device has not been evaluated in the public health insurance system for reimbursement. The MITRA-FR trial was therefore designed in order to provide a higher level of evidence of the device efficacy with funding from the French Health Ministry.
In contrast, the RESHAPE-HF trial had been initiated within European countries, such as Germany, where the MitraClip system is already reimbursed and used in routine practice. In the United States, the COAPT trial is aimed at showing the device efficacy to support the FDA approval in patients with secondary MR. Both trials are fully sponsored by the MitraClip manufacturing company whereas the MITRA-FR trial has an academic sponsor.
| Impact on daily practice pMVR with the MitraClip system is a promising option in patients with symptomatic severe secondary MR despite optimal medical therapy who are judged inoperable or at high surgical risk. The impact of pMVR on mortality and morbidity in this population has to be assessed with prospective, comparative, randomised studies such as the MITRA-FR. This study, and the COAPT trials, will provide the highest level of evidence of the device efficacy. |
Funding
This study is being supported mainly by a grant from the French Ministry of Health (PHRC 2012, ref: 12-027-0355). The study sponsor also received research support from Abbott Vascular France. The study protocol was designed and written by the Steering Committee independently from Abbott Vascular France.
Conflict of interest statement
J-F. Obadia is on the advisory boards of and received consultant fees from Landager and St. Jude Medical. X. Armoiry has been invited as a congress delegate by Daiichi Sankyo. B. Iung has received consultant fees from Abbott, Boehringer Ingelheim and Valtech, and speaker’s fees from Edwards Lifesciences. T. Lefèvre has received minor fees from Abbott Vascular. N. Mewton has received consulting fees from Servier, and travel support from Novartis, Servier, and BMS. D. Messika-Zeitoun has received consulting and lectures fees from Abbott, Valtech and Edwards. J-N. Trochu has received consulting and lecture fees from Abbott. A. Vahanian is on the advisory board of Abbott Vascular and Valtech. The other authors have no conflicts of interest to declare.
Online Appendix 1. Primary outcome definition
Outcome: All-cause mortality
Definition: Defined as the occurrence of all-cause death
Outcome: Unplanned hospitalisations for heart failure
Definition: Any presentation at a hospital or urgent treatment centre requiring completion of the hospital admission procedures or equivalent and/or at least a 24-hour stay or until death of the patient
OR
An unplanned treatment given in an outpatient setting in which an IV diuretic and/or IV vasodilator and/or IV inotrope is administered
AND presence of all the following criteria:
a) New or increased symptoms of heart failure (e.g., shortness of breath/dyspnoea on exertion, paroxysmal nocturnal dyspnoea, orthopnoea, fatigue/reduced exercise tolerance, pulmonary oedema, jugular venous distension, hepatojugular reflux, altered haemodynamics, peripheral oedema, cardiomegaly)
b) New or increasing signs of heart failure including signs of fluid retention
Online Appendix 2. List of committees and members, participating centres and investigators (as of December 2014)
1. STEERING COMMITTEE
Pr Jean-François Obadia; Dr Xavier Armoiry; Pr Frédéric Collart; Pr Jean-Philippe Collet; Dr Bertrand Cormier; Pr Bernard Iung; Dr Thierry Lefèvre; Pr Jean-Noël Trochu; Pr Alec Vahanian
2. DATA SAFETY AND MONITORING BOARD
Dr Jean-Christophe Eicher; Dr Silvy Laporte; Pr Marc Laskar
3. CLINICAL EVENTS COMMITTEE
Pr Eric Bonnefoy-Cudraz; Dr Raphaël Dauphin; Dr Guy Durand de Gevigney; Dr Laurent Francois; Dr Nathan Mewton; Dr Jacques Robin; Dr Hélène Thibault
4. ECHOCARDIOGRAPHY CORE LABORATORY
Dr Eric Brochet; Dr Bernard Cormier; Pr David Messika-Zeitoun
5. MITRA-FR INVESTIGATORS
Lyon, Hôpital Louis Pradel: Jean-François Obadia, Gilles Rioufol, Hélène Thibault, Martine Barthelet, Sophie Thivolet
Paris, Hôpital Bichat: Alec Vahanian, Dominique Himbert, Patrick Nataf, Bernard Iung, Eric Brochet
Massy, Institut Hospitalier Jacques Cartier - Institut Cardiovasculaire Paris Sud: Thierry Lefèvre, Mauro Romano, Bertrand Cormier
Nantes, CHU de Nantes: Patrice Guérin, Vincent Letocart, Jean-Noël Trochu, Olivier Baron, Nicolas Piriou, Thierry Letourneau
Rennes, Hôpital Pontchaillou: Marc Bedossa, Guillaume Leurent, Erwan Donal, Hervé Corbineau
Besançon, CHU de Besançon: Sidney Chocron, François Schiele, Nicolas Meneveau, Romain Chopard
Créteil, Hôpital Henri Mondor: Emmanuel Teiger, Jean-Luc Dubois-Rande, Clémence Antoine, Jean-Paul Couetil, Pascal Lim, Jean-Luc Monin, Thibault Damy, Soulef Guendouz, Stéphane Champagne, Laura Ernande
Le Plessis Robinson, Centre Chirurgical Marie Lannelongue: Alexandre Azmoun, Jean-Yves Angel, Philippe Brenot, Alban Baruteau, Michel Slama, Philippe Deleuze, François Raoux, Philippe Garcon
Lille, CHRU de Lille: Alain Prat, André Vincentelli, Thomas Modine, Francis Juthier, Eric Van Belle, Arnaud Sudre, Anne-Sophie Polge, Marjorie Richardson
Marseille, CHU de La Timone: Jean-Louis Bonnet, Mathieu Pankert, Nicolas Michel, Gilbert Habib, Erwan Salaun
Montpellier, CHU de Montpellier: Florence Leclercq, Jean-Christophe Macia, Bernard Albat, Stéphane Cade, Fréderic Cransac
Montpellier, Clinique Le Millénaire: Christophe Piot, Frank Raczka, Catherine Sportouch, Maxime Pons, Fabrice Francois
Nancy, CHU de Nancy-Brabois: Yves Juilliere, Christine Selton-Suty, Olivier Huttin, Thierry Folliguet
Pessac, CHU de Bordeaux: Lionel Leroux, Pierre Coste, Louis Labrousse, Stephane Lafitte, François Picard, Marina Dijos
Strasbourg, CHU de Strasbourg: Patrick Ohlmann, Jean-Georges Kretz, Arnaud Mommerot, Olivier Morel, Annie Trinh, Hélène Petit-Eisenmann, Paola Goette-Dimarco, Annie Samet
Toulouse, CHU de Toulouse: Nicolas Dumonteil, Mathieu Gautier, Bernard Marcheix
Marseille, Hôpital Saint-Joseph: Rémi Houel, Patrick Joly, Jacques Bille, Philippe Commeau, Emmanuel Philip, Nicolas Michel
Clermont-Ferrand, CHU Gabriel-Montpied: Pascal Motreff, Azarnoush Kasra, Charles Vorilhon, Guillaume Clerfond
Grenoble, CHU La Tronche: Bernard Bertrand, Hélène Bouvaist, Carole Saunier
Angers, CHU d’Angers: Frédéric Pinaud, Frédéric Rouleau, Thomas Benard, Alain Furber, Jean-Louis Debrux
Le Chesnay, Hôpital Privé de Parly 2: Claude Vaislic, Xavier Favereau, Philippe Maribas
Toulouse, Clinique Pasteur: Didier Tchetche, Bruno Farah, Olivier Fondard, Atul Pathak, Laurent Bonfils, Issam Abouliatim

