
The different aetiologies of mitral regurgitation (MR) have a direct bearing on its prognostic impact and the selection and timing of treatment. Simply speaking, MR may be classified as degenerative (DMR) or functional (FMR), representing primary disease of the mitral valve (MV) or the left ventricle (LV), respectively. Surgical repair of severe DMR by experienced surgeons has high success rates with acceptable periprocedural morbidity and excellent durability, returning patients to their age/sex-matched expected survival. As such, surgical MV repair has a class I recommendation to treat severe DMR in the EU and US guidelines1,2, although with level of evidence B as no randomised controlled trials (RCTs) have been performed. In surgically eligible patients, the EVEREST II RCT demonstrated that MV surgery is more effective than transcatheter mitral leaflet approximation with the MitraClip in severe DMR, although registry studies have demonstrated that the MitraClip may provide symptomatic benefit for patients with severe DMR who are at prohibitive surgical risk (class IIb in the guidelines)1,2. Medical therapy is palliative at best in patients with structural MV disease.
In FMR, apical and lateral distraction of the papillary muscles due to global or regional LV dilatation results in tethering and lack of coaptation of otherwise normal mitral leaflets. Treatment of patients with heart failure (HF) and FMR is much more complex than DMR as the symptoms and prognosis are, to a large extent, determined by the underlying LV dysfunction. Nonetheless, the additional volume overload from quantitatively severe FMR is an independent predictor of mortality and HF hospitalisation3. HF medications and cardiac resynchronisation therapy (CRT) in select cases are the only class I recommended therapies for FMR1,2. No RCTs of MV surgery compared with medical therapy in severe FMR have been performed. However, most observational studies using propensity-matching and other statistical techniques to adjust for baseline differences have not suggested a prognostic benefit of surgical MV repair in severe FMR, and the MR recurrence rate is high1,2,4. Chordal-sparing MV replacement provides a more durable correction for FMR4, but similarly has not been demonstrated to reduce death or hospitalisation. As such, MV surgery has a class IIb recommendation in international guidelines for isolated severe FMR treatment1,2.
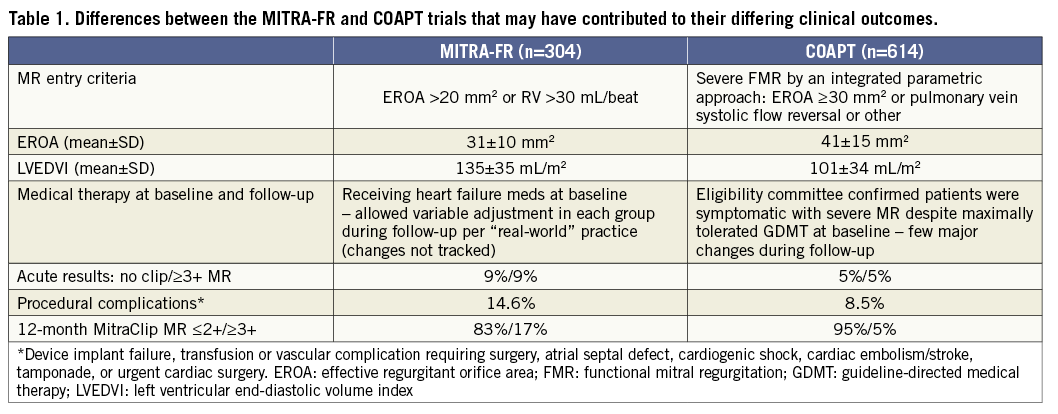
The MitraClip® (Abbott Vascular, Santa Clara, CA, USA) may provide symptomatic benefit in patients with FMR, and in Europe it is widely used for this purpose. Until recently, however, whether reduction of FMR with the MitraClip (or any therapy) provides prognostic benefit in HF was uncertain. Two RCTs of the MitraClip in HF patients with severe FMR compared with medical therapy have now recently been reported, reaching diametrically opposite conclusions. In the MITRA-FR trial, one-year rates of death or HF hospitalisation were not improved by the addition of MitraClip to background medical therapy, whereas in the COAPT trial the two-year rates of survival, freedom from HF hospitalisation, quality of life and exercise performance were markedly improved in MitraClip-treated patients compared with maximally tolerated guideline-directed medical therapy (GDMT) alone5,6. In both studies MitraClip therapy was safe, with low rates of periprocedural complications. The differences between these trials may be explained by variations in the severity of MR and LV dilatation among the patients enrolled, background use and changes in medical therapies during follow-up, and technical and procedural outcomes of the MitraClip procedure (Table 1). Perhaps most importantly, patients enrolled in COAPT had substantially more severe MR but with smaller LV dimensions than those enrolled in MITRA-FR. As such, the MR component haemodynamically contributed more to the symptomatology and poor prognosis in COAPT than in MITRA-FR. To conceptualise this, Grayburn and colleagues have noted that most patients in COAPT had “disproportionately severe” MR whereas most patients in MITRA-FR had “proportionately severe” or non-severe MR7. Thus, these two trials have complementary value in that they inform which HF patients are likely or unlikely to benefit from transcatheter MV repair.
In the present issue of EuroIntervention, Kortlandt and colleagues report the mortality rates among 963 high-risk patients with 3+/4+ MR undergoing surgery, MitraClip treatment or conservative therapy at four Dutch centres8.
In patients with FMR (n=688), the multivariable-adjusted HR (95% CI) for mortality at median 2.8-year follow-up for conservative therapy (n=228) vs. MitraClip (n=365) was 1.79 (1.34 to 2.39), p<0.001, similar to the two-year HR (95% CI) for patients treated with GDMT alone vs. MitraClip plus GDMT in the COAPT trial (1.62 [1.22 to 2.17], p<0.001)6. Only 95 FMR patients were treated with MV surgery (80% repair). These patients were younger and in general had fewer comorbidities than those treated with either the MitraClip or medical therapy. The multivariable-adjusted mortality of MV surgery compared with MitraClip was not significantly different (HR 0.86, 95% CI: 0.54 to 1.38; p=0.54). Similar results were seen in propensity-adjusted models. Not surprisingly, patients with DMR (n=275) had fewer comorbidities and better LV function than those with FMR, although they were slightly older. DMR treatment was with MitraClip (n=165), surgery (n=66; 69% repair) or medical therapy only (n=44). There were substantial differences in baseline features between these groups (e.g., conservatively treated patients were oldest while MitraClip patients had the highest surgical risk by EuroSCORE II). Detailed analysis of survival by treatment among DMR patients was not performed due to this marked selection bias. Moreover, outcomes beyond mortality in both groups were not available (e.g., hospitalisation rates, quality of life), nor were long-term echocardiographic data examining LV remodelling or MR severity over time.
The limitations of non-randomised studies are well known and apply to the current report. In particular, the reasons why physicians choose surgery vs. MitraClip or elect to leave severe MR uncorrected are not documented in observational databases; even the most sophisticated statistical models cannot overcome residual confounding. In the present study, the MitraClip-treated patients were selected from 2009-2016, whereas the surgically and conservatively treated patients were from an earlier period (2007-2012, depending on MitraClip availability at each centre). This introduces further potential differences between the groups in background therapies or practices that evolve over time. In addition, the grading of MR severity can be challenging, with different criteria used in the EU and the USA7. No echocardiographic core laboratory was utilised, and the definition of 3+/4+ MR used in the present study and quantitative measures of MR among enrolled patients were not provided. It is very possible that a proportion of patients had less than severe MR, and whether the degree of MR was balanced between groups is uncertain. Thus, relying on the observed comparative outcome differences between therapies from the present study should be avoided. Such reports are useful at documenting relative frequencies of treatments, and in general the present study supports the short-term safety of both MitraClip and surgery in selected patients with severe MR, although intermediate-term mortality was substantial with all treatments, reflecting the underlying patient substrate.
Based on the totality of data, MV surgical repair should remain the standard of care for most patients with DMR, with MitraClip reserved for those in whom surgical repair is not feasible. Future randomised trials will demonstrate whether device and technique advances have improved MitraClip outcomes sufficiently such that they provide acceptable outcomes for intermediate surgical risk patients with DMR. As regards FMR, the implications of the COAPT and MITRA-FR studies are currently being considered and will inform labelling of the MitraClip for FMR by regulatory authorities and recommendations in future guideline updates. Based on the robust findings from the COAPT trial, in our opinion HF patients meeting strict COAPT criteria (3+/4+ MR graded using the integrated approach recommended by the American Society of Echocardiography9, LV ejection fraction 20-50%, LV end-systolic diameter ≤70 mm who remain symptomatic despite maximally tolerated GDMT including CRT if appropriate) should be offered the MitraClip if anatomically eligible to improve survival (one life saved per six patients treated over two years), prevent HF hospitalisations (one hospitalisation prevented per three patients treated over two years) and improve functional capacity and quality of life. Additional studies are required to determine whether surgical MV repair or replacement should be considered for select patients with isolated FMR. Further investigation is also required to define the role of the numerous emerging transcatheter MV repair and replacement devices in DMR and FMR, a complex calculus that will depend in part upon patient eligibility for surgery and the MitraClip. Even more fundamental questions relate to the optimal timing of MR treatment (intervening early, or conversely before it is too late), whether select patients with moderate MR may benefit, and examining whether MR reduction is useful in cardiogenic shock or the stage D patient (pre-LV assist device or transplant). What can be stated with certainty is that advances in transcatheter and surgical approaches buttressed by evidence-based medicine will continue to improve outcomes for high-risk patients with severe MR.
Conflict of interest statement
G. Stone has served as a consultant to Claret, Medical Development Technologies, Backbeat, Sirtex, Matrizyme, Miracor, Neovasc, V-wave, Shockwave, Valfix, TherOx, Reva, Vascular Dynamics, Robocath, HeartFlow, Gore, Ablative Solutions, Abiomed and Ancora; has received speaker honoraria from Terumo and Amaranth; has equity/options in Ancora, Cagent, Qool Therapeutics, Aria, Caliber, MedFocus family of funds, Biostar family of funds, Applied Therapeutics and SpectraWAVE; is a director of SpectraWave; and his employer, Columbia University, receives royalties for sale of the MitraClip from Abbott. M. Mack has served as the co-principal investigator of the COAPT (Abbott) and PARTNER 3 (Edwards Lifesciences) trials and as the study chair of the APOLLO trial (Medtronic).

