
Abstract
Aims: Although mitral cerclage annuloplasty can reduce mitral regurgitation, the potential risks for erosion of the surrounding tissue or conduction blockage are barriers to human translation. This preclinical study aimed to provide a proof of concept for a novel approach, mitral loop cerclage (MLC), designed to address these shortcomings.
Methods and results: MLC consists of: 1) a novel appliance termed a coronary sinus and tricuspid valve protective device (CSTV) that includes a tension locker, and 2) a nylon-coated, braided stainless steel rope (0.6 mm thick) with a coronary artery protective device in a single unit (cerclage rope). Nine healthy farm swine underwent MLC in short-term (two weeks, n=4) and midterm (six weeks, n=5) survival experiments under X-ray fluoroscopic guidance imaging. The procedural success rate was 100%. MLC resulted in a significant reduction of the septal lateral dimension of the mitral annulus (24.58±2.16 vs. 21.26±1.43 mm, p=0.04) and left ventricular (LV) volume in diastole (75.9±3.9 vs. 70.6±5.0 ml, p=0.04) in the midterm group. No conduction abnormalities or serious complications were noted beyond trivial tricuspid regurgitation in all cases (n=9). Necropsy showed no evidence of tissue erosion and an excellent biocompatibility of the implanted devices.
Conclusions: MLC, as a novel approach for catheter-based mitral valve repair, appeared feasible in this short-term preclinical model. Further studies with longer follow-up in a cardiomyopathic animal model are needed to verify the clinical feasibility and safety of MLC.
Abbreviations
CS: coronary sinus
CSTV: coronary sinus and tricuspid valve protective device
CT: computed tomography
ECG: electrocardiogram
ePTFE: expanded polytetrafluoroethylene
IVC: inferior vena cava
LA: left atrium
LAD: left anterior descending artery
LV: left ventricular
MLC: mitral loop cerclage
MV: mitral valve
RV: right ventricle
SVC: superior vena cava
TTE: transthoracic echocardiogram
TV: tricuspid valve
Introduction
Percutaneous mitral valve (MV) repair attracts much attention in interventional cardiology because it has shown excellent efficacy for improving the quality of life of heart failure patients who have significant mitral regurgitation1-3. Among the many technologies being developed, coronary sinus (CS) annuloplasty has the obvious advantage of an easy approach with a simple procedure, in spite of two major limitations1,4. The first is that it shows only modest efficacy5,6. The second is the potential for compromising the underlying coronary artery7. The modest efficacy of CS approaches may result mainly from the inherent limitation of the hemi-circumferential tension design itself. Among devices used for CS approaches, mitral cerclage annuloplasty offers a new method that could overcome these limitations8. However, it poses other risks such as the potential for damaging tricuspid valve (TV) function and conduction blockage by its persistent mechanical collision with the underlying contacting tissue, especially at the right ventricular (RV) basal septum and the TV that is to be entrapped by the cerclage suture.
We have designed a coronary sinus and tricuspid valve protective device (CSTV) for preventing the shortcomings of mitral cerclage. Using this device, the tension is indirectly delivered through a loop by the CSTV, rather than applying direct cerclage tension to the underlying myocardial tissue. This novel design preserves the advantageous circumferential tension around the mitral annulus while avoiding the safety issues of mitral cerclage.
In the present study, we investigated the proof of concept of mitral loop cerclage (MLC) annuloplasty with the CSTV through a preclinical study.
Methods
ANIMALS
Animal experiments were approved by the PNUYH Animal Care and Use Committee. Healthy Yorkshire farm swine (62±17 kg) were used for technical development (n=42), short-term (two weeks, n=4), and midterm survival experiments (six weeks, n=5). Anaesthesia was induced with atropine, butorphanol, ketamine, and xylazine, and maintained with inhaled isofluorane and mechanical ventilation.
THE CONCEPT OF MLC AND THE CSTV
The main concept of MLC is delivering cerclage tension through a loop made by the CSTV. The MLC procedure consists of two parts. One is creating a path of circumferential tension around the MV annulus using a specially designed 0.6 mm thick radiopaque metallic rope (cerclage rope). The other part is delivering the CSTV through the cerclage rope.
The CSTV was designed as shown in Figure 1. It comprises a CS tube and a TV tube, which are hollow cylindrical tubes for protecting tissues of the CS or TV, respectively, and a stem through which the CS and TV tubes extend to be fixed and combined at its hinge portion. Each tube is made of polyurethane coated with ePTFE and is 5 Fr in size. Tension locking was performed at the tip of the CSTV stem with a specially designed tension stopper. The TV tube has a ring-shaped stopper (RV exit stopper) to prevent further advancement of the TV tube into the interventricular septum. Furthermore, the length of the TV tube (specifically the lengths of the RV exit stopper and the hinge) was intentionally designed to be longer than the distance from the RV exit to the orifice of the CS. The hinge where the CS and TV tubes meet is automatically placed near the orifice of the CS. When tension is applied through the cerclage rope, the TV tube is to be fixed to the heart at its hinge and at the point of the RV exit stopper. Then, the TV tube is suspended freely in a “C” shape without being directly attached to the TV tissue. Such a technique can avoid TV tissue damage (erosion) as well as conduction system damage that could occur with direct contact of the cerclage rope to the underlying basal septal area (Figure 1).
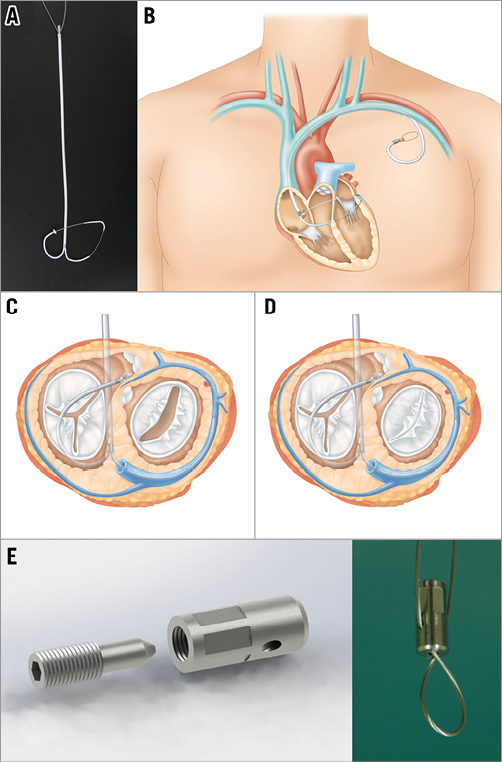
Figure 1. The design and function of the CSTV and MLC. A) The mature CSTV device for human translation. B) MLC for human translation. C) & D) The function of the CSTV. E) The locking device.
OTHER DEVICES FOR MLC
MLC requires several devices developed by Tau-PNU Medical Co., Busan, South Korea, along with other conventional interventional tools, such as various 0.014” PTCA guidewires and a balloon-tipped guiding catheter (Cello™ 8 Fr; ev3/Covidien, Plymouth, MN, USA).
1. The cerclage rope
The cerclage rope is made of braided fine stainless steel wire with a nylon coating (Nylon coated SUS 304, ![]() 0.5-0.6, 1×19 strands). Its metallic component makes the rope radiopaque. The rope has a coronary artery protective section consisting of a rigid metallic arch (Figure 2).
0.5-0.6, 1×19 strands). Its metallic component makes the rope radiopaque. The rope has a coronary artery protective section consisting of a rigid metallic arch (Figure 2).
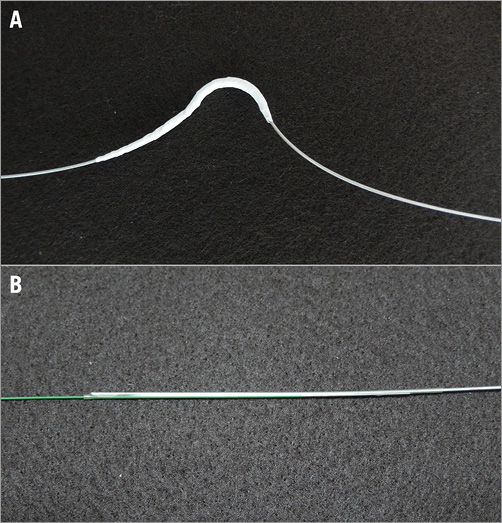
Figure 2. The cerclage rope. A) The coronary artery protective section of the cerclage rope on a single unit. B) Instant connection of the cerclage rope with the 0.014” guidewire by a biocompatible heat shrinkage tube.
2. The device for RV guidewire capture (RV wire capture)
This device is specially designed for capturing the 0.014” guidewire which exits into the RV cavity after traversing the basal interventricular septum and CS. In order to capture the wire without chordal or adjacent tissue entrapment in the RV, the wire should be pulled out only within a particular space, termed the “safe zone”. The safe zone is defined as an imaginary enclosed circular space bordered by: 1) the TV leaflet and its subvalvular structures such as the chordae of the TV and the papillary muscle, and 2) the moderator band. For securing this safe zone, a semi-rigid curved catheter (safe zone catheter, 6 Fr) with an inflatable balloon (8 mm in diameter) on its tip is introduced from the femoral vein to the pulmonary artery, and then a 0.035” stiff wire is further advanced to the distal pulmonary artery through its central lumen for strong support. The balloon prevents its path from including the narrow space between the chordae of the TV. Furthermore, the approach from the inferior vena cava (IVC) to the pulmonary artery with a semi-rigid catheter avoids the space between the moderator band and the RV apex. Once the safe zone has been secured by these methods, the capturing part of the safe zone catheter is introduced. This part contains mesh with a central lumen for the stiff wire (0.035”) (Figure 3).
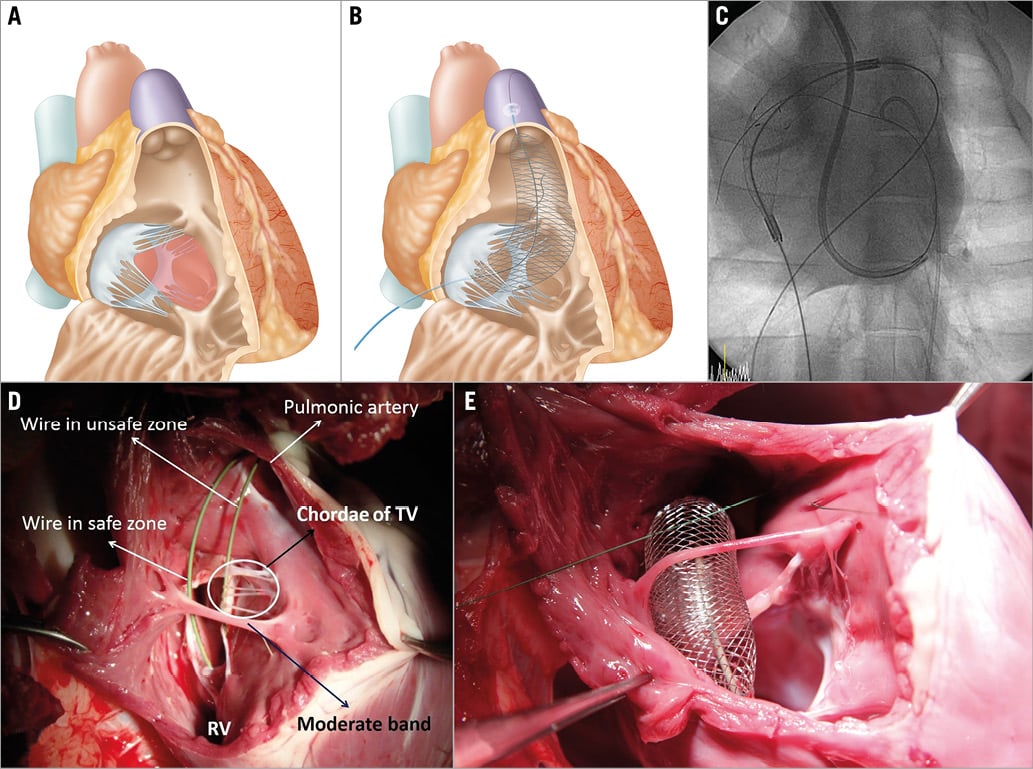
Figure 3. The safe zone and RV wire catcher. A) The safe zone is indicated in red. B) Mesh type RV wire catcher with a central lumen to keep the stiff wire in the safe zone. C) A guidewire from the coronary sinus exits into the RV cavity and then into the RV wire catcher. D) & E) Necropsy findings.
PROCEDURE OVERVIEW
For MLC, 18 Fr introducer sheaths were placed in the right jugular and femoral veins, and 6 Fr introducer sheaths were placed in one of the femoral arteries. The balloon-tipped guiding catheter (Cello 8 Fr) was engaged into the CS to perform a pressurised venogram. The method used for traversing the interventricular septum and re-entering the RV cavity with a conventional 0.014” PTCA wire was identical to that used for mitral cerclage. Among several septal veins, basal septal veins are preferably selected to engage the basal part of the interventricular septum (Figure 4A). Once the wire is engaged into a septal vein, gentle pushing of the wire along the vein with multiple rotational manoeuvres provokes simultaneous ventricular premature contractions (VPC). These VPC together with a wire position beneath the left anterior descending artery by coronary angiography were regarded as sure signs that the wire was within the interventricular septal wall. Although any 0.014” PTCA wire will work for septal traversal, stiffer wires, such as the MIRACLEbros, Conquest wire or even the Astato wire (all ASAHI Intecc, Aichi, Japan), were very helpful in this step. The wire manipulation technique for traversing the interventricular septum was quite similar to that of the chronic total occlusion (CTO) procedure for coronary intervention. In most cases of wire manipulation, the wire is prone to move towards the septum of the RV inflow tract (RVIT) along with the course of the septal vein, rather than towards the RV outflow tract (RVOT), which is regarded as a more suitable exit site. Therefore, redirection of the wire while traversing the septum was needed in most cases. For this purpose, a double lumen microcatheter (Crusade; Kaneka Medix Corp., Tokyo, Japan) is very useful, as it actually enables a second wire to be introduced easily into the septal wall through its side lumen at a certain good point of the septum of the first wire track. Furthermore, a double lumen microcatheter provides excellent back-up support (Figure 4B). A mesh-type RV wire catcher from the femoral vein was also placed along the space between the pulmonary valve and right atrium. The expanded mesh of the RV wire catcher, especially the upper half of it, becomes a good landmark of the RVOT cavity under fluoroscopic guidance imaging, which helps direct septal traversal of the wire. When the wire had exited into the RV cavity, catching the wire by folding the mesh indicated that the wire had successfully exited into the target site (Figure 4C). Then, the wire was pulled down into the inferior vena cava so that the guidewire could exit the heart after encircling the basal left ventricle, which includes the perimitral annulus (Figure 4D). After this step, the guidewire was replaced with the cerclage rope using a microcatheter (Progreat® Ω 2.8 Fr; Terumo Corp., Tokyo, Japan) or by instantly connecting both ends of the guidewire with a biocompatible heat shrink tube (polyether/polyamide). The segment of the cerclage rope designed to protect the coronary artery was properly positioned under coronary angiography guidance (Figure 4E). Then, the CSTV was delivered over both ends of the cerclage rope after pulling the other end of the femoral site into the neck vein with a snare (Figure 4F, Figure 4G). After the CSTV settled into place naturally, tension was delivered at the proximal tip of the CSTV outside of the body by gentle manual pulling of the cerclage rope under imaging guidance via a transthoracic echocardiogram (TTE) (Figure 4H). The tension at the tip of the CSTV was locked with a specially designed tension locker (Figure 1). The portion of the CSTV with the tension locker that is outside of the body was embedded under the subcutaneous tissue of the upper chest wall, similar to the placement of a permanent pacemaker (pacemaker type MLC).
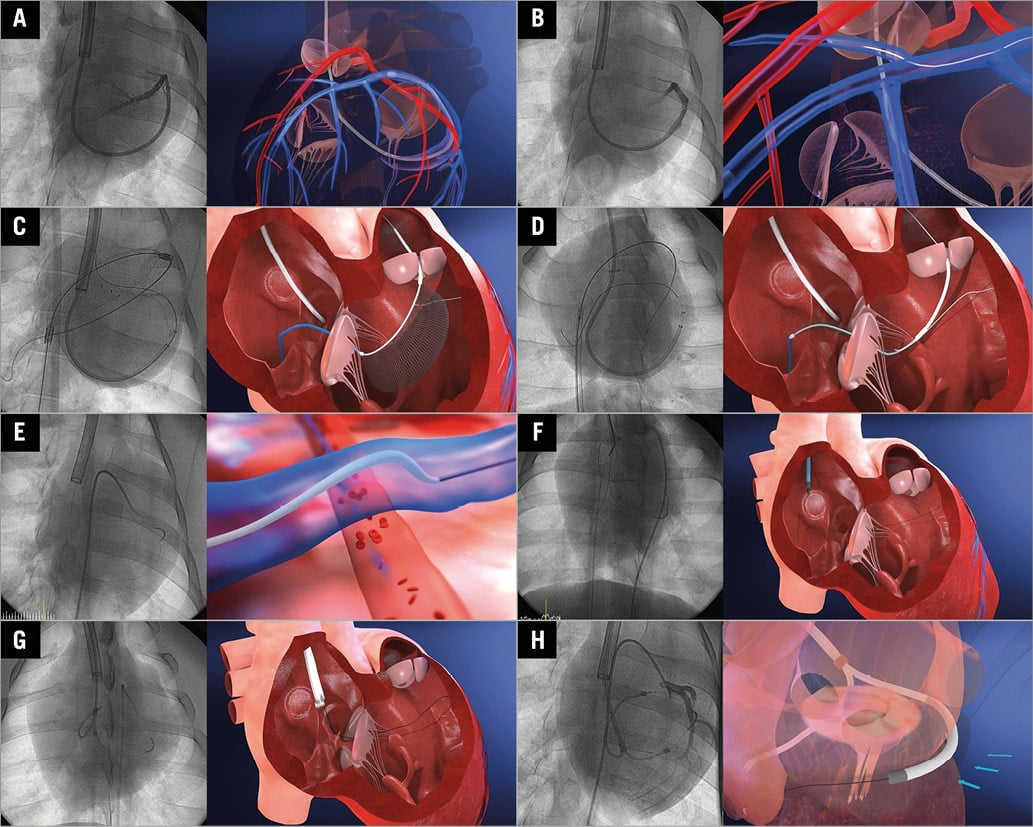
Figure 4. Procedure overview. A) Pressurised venogram with a balloon-tipped guiding catheter. B) The 0.014” guidewire traversing the basal interventricular septum via a selected septal vein. C) RV wire catcher for grasping the guidewire. D) Pulling down the wire into the inferior vena cava. E) Positioning of the segment of the cerclage rope designed to protect the coronary artery. F) Pulling the other end of the femoral site into the neck vein with a snare. G) Delivery and positioning of the CSTV. H) Tension was delivered by pulling of the cerclage rope under imaging guidance.
IMAGING MODALITY AND MEASUREMENTS
The present study used cardiac computed tomography (CT) (128-slice dual source CT, SOMATOM® Definition Flash CT scanner; Siemens Medical Solutions, Forchheim, Germany), TTE (ACUSON Cypress™; Siemens Medical Solutions), and X-ray fluoroscopy (Integris H5000F; Philips Medical Systems, Best, The Netherlands). Cardiac CT images were obtained before the procedure (baseline, n=9) and at the last follow-up (two weeks in the short-term follow-up group [n=4] and six weeks in the midterm follow-up group [n=5]) The protocol for CT was as follows: 140 kVp, 320 effective mAs using a continuous helical scan, a rotation time of 280 msec, slice collimation of 2×128×0.6 mm, and 120 mL of iopromide (Ultravist® 370; Bayer Schering Pharma AG, Berlin, Germany) administered at a rate of 5 ml/sec followed by 20 mL saline at the same rate.
TTE was used to assess how much of the septal lateral dimension of the mitral annulus was reduced during tension application in the beating heart. Tricuspid regurgitation was also assessed using TTE. X-ray fluoroscopy and coronary angiography was used to guide the whole procedure. During wire manipulation, we ensured that the guidewire passed through an area beneath the left anterior descending artery.
Most parameters were defined and measured according to general echocardiographic criteria. However, the following parameters were defined and measured according to our study protocol:
1. Left ventricular (LV) volumetric analysis of cardiac CT imaging: for volumetric analysis, all CT data were transferred to dedicated software (Aquarius iNtuition™; TeraRecon, Tokyo, Japan) and reconstructed at 10-100% of the R-R interval in 10% increments (slice thickness: 0.7 mm, slice width: 0.4 mm)9. Thereafter, LV function and volume were evaluated by a cardiac radiologist using dedicated software with a semi-automated LV endocardial and epicardial contour detection.
2. Measurement of LV base: the length of the LV base was defined as the distance from the aorto-LV or LA-LV junction on the three-chamber view and the distance between both LA-LV junctions in the four-chamber view.
ENDPOINTS OF THE ANIMAL STUDY FOR EACH STEP
1. Non-survival ex vivo and in vivo tests for CSTV function.
A total of 44 farm swine were used to develop this technique. In a subset study, CSTV function was tested to determine how much of the mitral annulus could be reduced in a harvested heart (n=4) and a beating heart (n=1). Retrieval of all devices was also tested after completion of MLC in a beating heart (n=2).
2. Short-term (two weeks, n=4) and midterm (six weeks, n=5) survival experiments.
A short-term survival experiment after MLC with an immature prototype device was performed to test the following endpoints: 1) whether the coronary artery protective device remained in place without migration, 2) whether the function of the TV was intact, 3) whether any signs of conduction abnormality were observed on ECG, and 4) whether the procedure was feasible. Midterm survival experiments were performed to test the same endpoints with a mature CSTV device that was coated with biocompatible material (e-PTFE). In the midterm survival group, the tension was intentionally applied until the septal lateral dimension of the mitral annulus was reduced by approximately 20% under the guidance of TTE in the three-chamber view. During follow-up, TTE was used in the first week of follow-up in the midterm group. Both cardiac CT and TTE were used at the last follow-up in all survival groups. X-ray fluoroscopy including coronary angiography was performed at every step of the follow-up. In the midterm group, tension release was tested by unlocking the tension stopper in an animal at the six-week follow-up.
All hearts of the midterm group were harvested and carefully assessed visually. Pathologic analysis of MLC was performed with randomly selected harvested hearts from the midterm group (n=2). The samples were sent to a lab for tissue preparation (Genoss Co., Ltd. Suwon, South Korea), and a pathology report was given by an experienced pathologist.
Statistics
Statistical verification was conducted using the Statistical Package for the Social Sciences, Version 21 (IBM Corp., Armonk, NY, USA). All parameters were compared using a Wilcoxon signed-rank test. The Friedman test was also performed for echocardiographic parameters. Parameters are reported as the mean±SD, and a p-value <0.05 was considered significant.
Results
PROCEDURAL FEASIBILITY AND COMPLICATIONS IN THE SURVIVAL EXPERIMENTS
All procedures were successful in the short-term (n=4) and midterm (n=5) survival experiments. One animal which did not undergo MLC due to the absence of the great cardiac and anterior interventricular vein was excluded from the data analysis. The mean whole procedural time, from vascular access to skin closure, was 154±44.5 minutes.
In all cases, only mild tricuspid regurgitation (grade I) was noted during follow-up. Conduction abnormalities of any kind were not seen in any animals.
A transient ST-segment change without any sign of a compromised coronary artery was noted in one case (11.1%) when tension was applied. Apical ballooning suggestive of LV dysfunction was also noted in this case. These signs appeared to be dependent on tension application. However, an abnormal ECG sign and LV dysfunction were not found at the one-week follow-up or thereafter with the same tension applied during the six-week survival experiment. This finding was not observed in our technical development phase (n=44).
Mild dissection of the great cardiac vein due to forceful guiding of the catheter or guidewire manipulation was noted in three cases (33.3%).
Mild temporary contrast dye staining due to guidewire manipulation was noted in one case (11.1%) around the area of the basal septum. Nonetheless, this did not lead to regional LV dysfunction.
Mild pericardial effusion was seen in one case (11.1%) due to passage of the 0.014” guidewire into the pericardial space during basal septal traversal.
THE FUNCTION OF THE 0.014” GUIDEWIRE CATCHER IN THE RIGHT VENTRICLE (RV WIRE CATCHER)
With the use of the RV wire catcher, all guidewires from the CS and septal traversal were safely passed through the safe zone without causing any damage to the surrounding tissue in the technical development experiments and the survival groups (Figure 3).
THE FEASIBILITY OF MITRAL LOOP CERCLAGE
INTERACTIVE TENSION ADJUSTMENT THROUGH THE CSTV
After the MLC procedure, tension could be applied to beating hearts under TTE guidance for reducing the septal lateral dimension by approximately 20% (midterm survival group, n=5) or to arbitrary points (short-term group, n=4). In experiments with a beating heart, the tension through the CSTV was able to reduce the septal lateral dimension as much as approximately 40% (Figure 5).
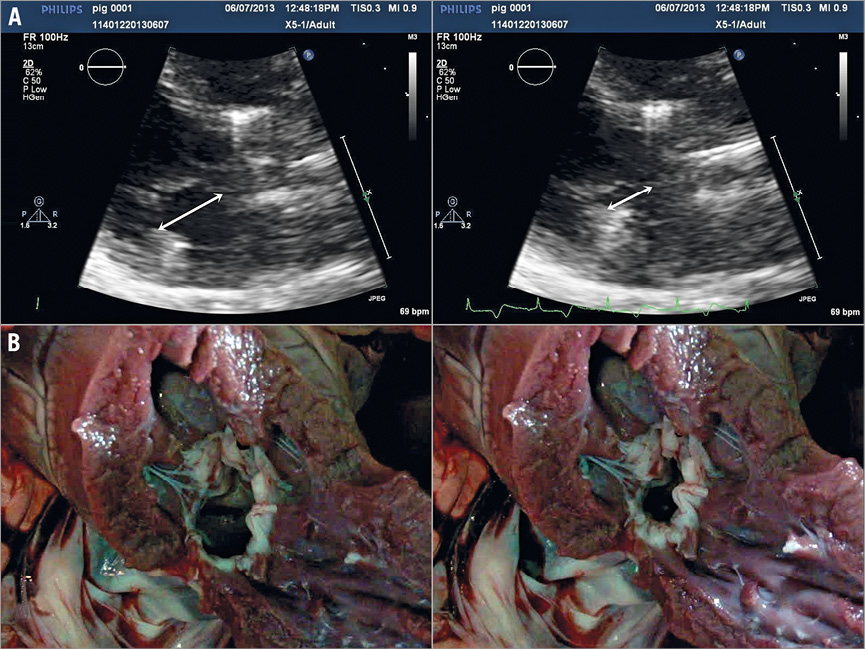
Figure 5. Interactive tension adjustment of the coronary sinus and tricuspid valve protective device (CSTV). A) Tension delivered on a beating heart through the CSTV reduced the septal lateral length (arrow) of the mitral annulus by 42% (19 mm to 11 mm). B) Tension application through the CSTV of a harvested heart.
THE PROTECTIVE ROLE OF THE TV TUBE IN THE CSTV
The TV tube of the CSTV formed an arch that was freely suspended in the RV cavity in all survival and non-survival experiments without coming into direct contact with the TV, subvalvular apparatus, and basal interventricular septum of the RV wall (Figure 6, Figure 7).
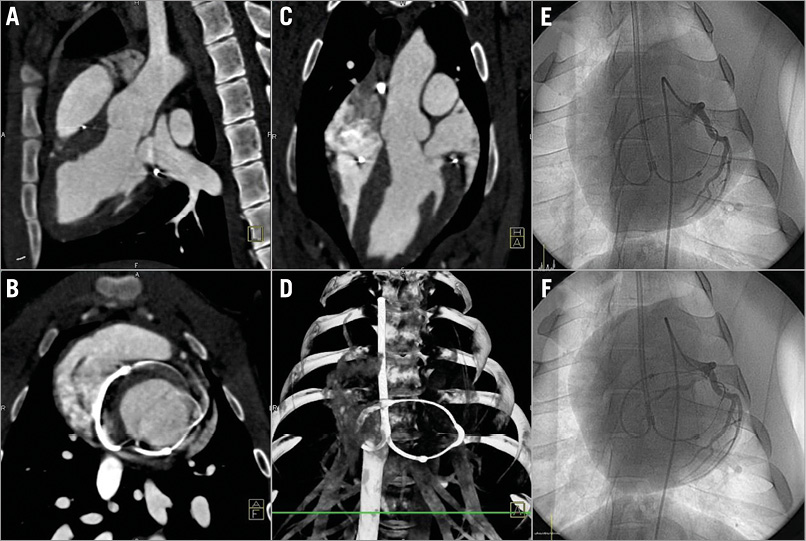
Figure 6. A six-week follow-up CAG and cardiac computed tomography. A) - D) A representative case of six-week follow-up cardiac computed tomography. E) & F) Tension release at the six-week follow-up showed mild loosening of the space between the left circumflex artery and the coronary artery protective device.
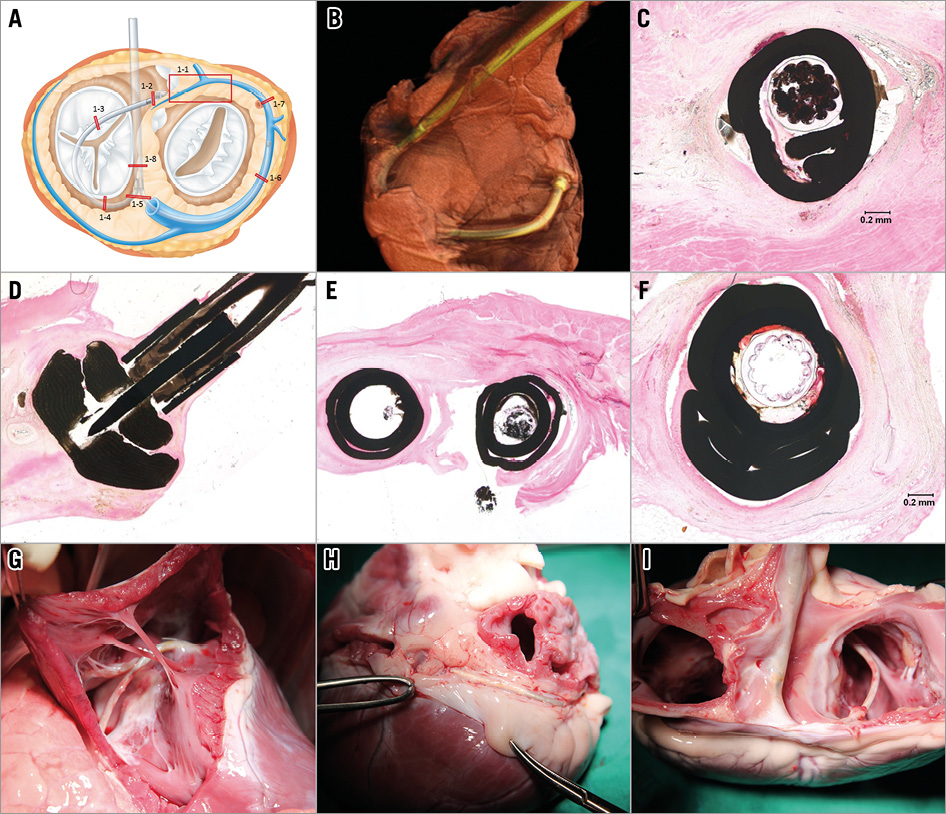
Figure 7. A harvested heart at six weeks and pathological examination. A) - F) Pathological examination revealed excellent biocompatibility of the implanted device without any evident erosion. In the harvested heart at six weeks: TV tube of the CSTV (G); coronary artery protective device of the cerclage rope (circle) that is firmly fixed in place by adhesion to the roof of the coronary sinus (H); posterior aspect after removing both atria (I).
THE CHANGE IN MV GEOMETRY AND LV FUNCTION AFTER MLC
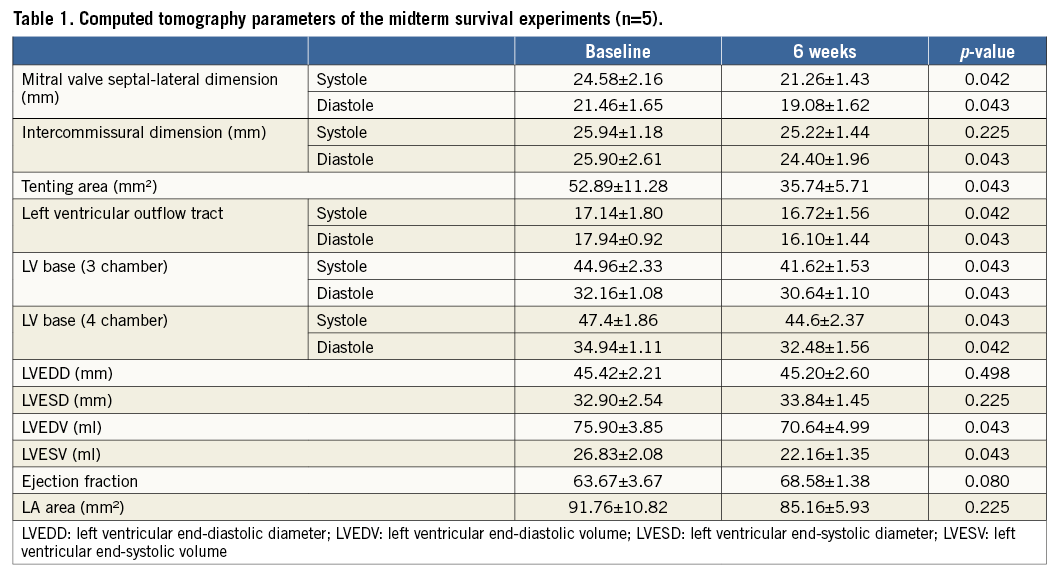
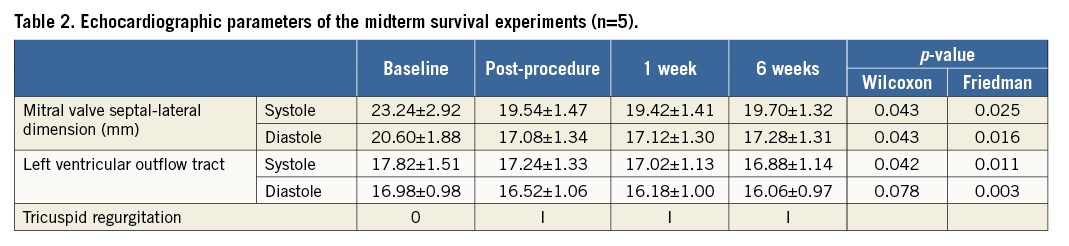
MLC had a favourable impact on MV geometry and enabled better coaptation. The septal lateral dimension of the mitral annulus and mitral tenting area were significantly reduced. In vitro animal tests (n=4) showed that applied tension through mitral loop cerclage changes the septal lateral dimension more than the intercommissural dimension of the mitral annulus. While the septal lateral dimension was decreased with mild tension, the intercommissural dimension only started to decrease with moderate to severe tension and at a slower rate than the change in the septal lateral dimension8,10 (Figure 8, Figure 9). The LV ejection fraction was not reduced (Table 1, Table 2).
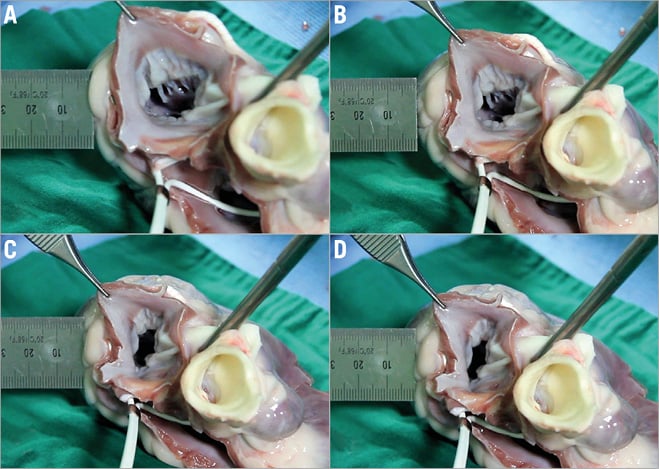
Figure 8. Effect of graded tension on the annular dimensions. A) - D) With progressive tension, while the annular septal-lateral dimension (SLD) was decreased with mild tension, the intercommisural dimension (ICD) only started to decrease with moderate to severe tension.
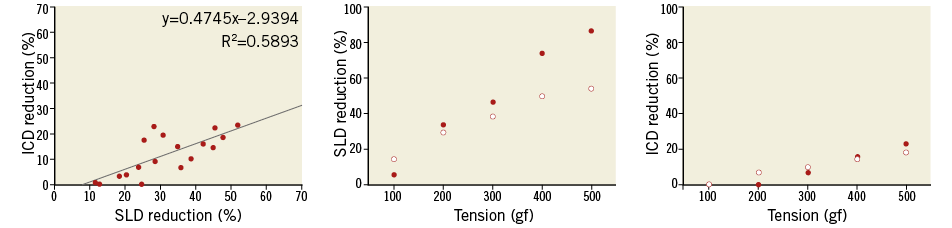
Figure 9. Correlation of SLD and ICD reduction. There was a positive linear correlation in SLD and ICD reduction. However, ICD reduction was not evident in mild tension and showed a slower rate than the change in SLD.
THE REVERSIBILITY OF MLC TENSION
In the technical development phase, retrieval of whole devices (n=2) was possible by simply pulling out the CSTV and then the cerclage rope without leaving any abnormal function or damage to the heart. In one midterm follow-up animal, tension release was attempted after six weeks by simple exploration of the embedded tip of the CSTV in the subcutaneous tissue. It displayed a loosening of the mitral loop encircling the left ventricular base (Figure 6).
VISUAL AND PATHOLOGIC EXAMINATION OF HARVESTED HEARTS
The visual examination of all harvested hearts showed no evidence of tissue erosion. All parts of the devices were well covered by thin membranous tissue. Pathologic analysis showed consistent results without any significant sign of inflammation or evident erosion (Figure 7).
Discussion
In the present study, MLC demonstrated several advantageous features in addition to favourable morphological change of the mitral annulus: 1) MLC delivers circumferential tension around the mitral annulus via loop-mediated tension using the CSTV; 2) interactive delivery of optimal tension under imaging guidance can be performed in beating hearts; 3) MLC does not require sophisticated imaging tools such as 3D-TEE, with the exception of fluoroscopic X-ray angiography; 4) the tension can easily be readjusted later if needed, and 5) retrieval of all devices is feasible at an early phase.
In mitral cerclage annuloplasty, the basal interventricular septum and the septal leaflet of the TV (a half circle) are the areas of concern because damage to these areas can lead to significant tricuspid regurgitation or a conduction block. This study demonstrated that CSTV could prevent these potential complications while keeping circumferential tension on the CS. However, we still observed a mild increase in tricuspid regurgitation, and we cannot preclude the possibility that significant TR might occur in some patients with this therapy.
Another feature of MLC in this study is its tension locking system, which is at the proximal end of the stem of the CSTV tube. The design of MLC allows the optimal tension to be delivered gradually into the beating heart under interactive real-time imaging guidance until mitral regurgitation disappears. Tension locking can be performed in the SVC (intravascular type) or outside of the body (pacemaker-type) depending on the length of the CSTV stem. With the pacemaker-type tension fixation in this study, tension can easily be readjusted, if necessary, by simply revisiting the tension locking system with a small skin incision. This mechanism is an important emergency option if the MLC eventually squeezes the heart excessively or pinches the underlying coronary artery during the follow-up period, which could occur due to progressive LV remodelling in spite of treatment.
Other discussion points are the following.
1. Interventricular septal traversal and the concept of a safe zone in the RV cavity.
A. Interventricular septal traversal: interventricular septal traversal with a guidewire is the most challenging aspect of this procedure. Usually, the septal length that must be traversed by the wire is approximately 2 cm (data not shown). In all experiments, wire manipulation was performed under only X-ray fluoroscopic guidance. In the case of a non-visible basal septal vein even with pressurised venography, blind wire manipulation with a stiff wire was successful for penetrating into the basal septal myocardium in most cases.
B. The concept of a safe zone in the RV cavity.
When the guidewire has passed through the SVC–coronary sinus–basal interventricular septum and then exited into the RV cavity, safely securing the wire to pull it into the IVC is the most important step of the MLC. Otherwise, it could damage the chordae of the TV or the moderator band, which may result in serious complications. In order to avoid these adverse events, grasping and pulling the guidewire should be performed within a specific space defined as the safe zone. The specially designed RV catcher worked very well for this purpose.
2. Potential complications.
In our survival experiments, no serious complications were found. However, potential complications were identified during the technical development phase: CS dissection and rupture, pericardial effusion and tamponade, entrapment of the subvalvular structure of the TV and its consequent tricuspid regurgitation, tissue erosion, myocardial haematoma, conduction block, pinching of the underlying coronary artery, infection and bleeding. Other unknown complications may exist. The CS tube of the CSTV naturally causes mild narrowing of the coronary sinus. However, a very interesting recent study showed that a coronary-sinus reducing device was associated with significant improvement in symptoms and quality of life in patients with refractory angina11.
3. Study limitations.
A. In this animal study, healthy hearts were used instead of a mitral regurgitation model. However, the mechanism of reducing the septal to lateral dimension of the mitral annulus is almost the same as that of mitral cerclage, which has already demonstrated excellent efficacy in the treatment of mitral regurgitation8.
B. A longer-term follow-up study was not performed because swine grow too quickly. In this study, the animals grew so quickly that their body weight increased by 15.7±2.9% in six weeks. This resulted in an increase in heart size. However, in an adult human, a sick heart would reduce its volume (reverse remodelling) when the mitral regurgitation is corrected12,13. An increasing heart size could diminish the protective ability of the coronary artery protective device, though this would not occur in the case of a reducing heart size. However, even with an increase of body weight, the coronary artery protective device showed an excellent ability to protect the underlying coronary artery without displacement in this study.
Conclusion
MLC, as a novel approach for catheter-based mitral valve repair, appears to be feasible, effective, and safe. This promising preclinical result may warrant human translation.
Funding
This study was supported by grants from Tau-PNU Medical Co. This work was also supported by a two-year research grant of Pusan National University, and clinical research grant from Pusan National University Yangsan Hospital.
| Impact on daily practice This study presents a novel method for mitral cerclage annuloplasty that could reduce risks to the patient. |
Conflict of interest statement
J.H. Kim and S.C. Sung are the founders and stockholders of Tau-PNU Medical Co. J.H. Kim also has a connection with intellectual properties of mitral loop cerclage annuloplasty. The other authors have no conflicts of interest to declare.

