
Abstract
Aims: Transcatheter mitral valve-in-ring (TMVIR) implantation with transcatheter heart valve (THV) prostheses can be performed in patients with recurrent mitral regurgitation (MR) following annuloplasty. However, an oval configuration and sometimes the rigidity of surgical rings can often lead to suboptimal THV expansion, resulting in considerable paravalvular or central leakage. Therefore, our aim was to develop an annuloplasty ring that fully adjusts to THV implantation.
Methods and results: A three-dimensional annuloplasty ring was separated into four pieces at defined locations, the sections were reconnected with heat-shrinkable tubing and rearranged into the original shape. A non-tear stainless steel circular cord of defined length was inserted into the ring’s sewing cuff to serve as a limiting structure for THV expansion. We implanted this ring in the mitral position into an isolated pig heart, deployed a THV into the ring, and investigated its function. Fluoroscopy showed that, upon THV deployment, the four breaking points of the ring separated as expected, and the ring expanded in a circular fashion to full expansion of the limiting cord. It securely anchored the THV to the ring, leaving no paravalvular gaps.
Conclusions: We developed an expandable mitral ring to which the THV attached without leakage. This may impact on the future design of annuloplasty rings. Further studies should evaluate the safety of increasing the perimeter of a mitral ring and its durability.
Abbreviations
Ao: aorta
LA: left atrium
LVOT: left ventricular outflow tract
MR: mitral regurgitation
PC: perfusion cannula
TAVI: transcatheter aortic valve implantation
THV: transcatheter heart valve
TMVIR: transcatheter mitral valve-in-ring
VIR: valve-in-ring
W: extra stiff guidewire
Introduction
Mitral regurgitation (MR) is a common valvular heart disease with a strikingly rising prevalence in the elderly population in western developed nations1. Therapeutic approaches to relieve MR include surgical valve replacement and reconstruction techniques, depending on the underlying disease (primary or secondary) and the reason for MR2. In case of ischaemic MR, remodelling processes of the left ventricle may lead to annular dilatation and papillary muscle displacement with consecutive tethering of the mitral leaflets, resulting in restricted closure and central regurgitation3. Surgical ring annuloplasty using various ring devices is currently the standard treatment option of choice, as these rings reduce the mitral annular area by shortening anterior-posterior dimensions4. However, their repair efficiency is limited by persistent or recurrent MR, especially in the case of functional ischaemic MR. The development of recurrent MR after surgical repair is estimated to be up to approximately 4% a year, and about 30% of patients with ischaemic cardiomyopathy develop recurrent severe MR within six months after surgical treatment4,5. In this high-risk population, redo surgery is associated with significant morbidity and mortality. Therefore, minimally invasive transcatheter strategies, such as those used to treat aortic and pulmonary valve diseases and congenital anomalies, are desirable6-9.
Due to the different shapes and material properties of mitral annuloplasty rings and transcatheter heart valves (THV), difficulties may arise from the configuration of these devices (for example, oval ring vs. round-shaped THV), causing paravalvular or central leakage of the implanted THV10-13. Another general issue of transcatheter mitral valve-in-ring (TMVIR) procedures is the inability to achieve the optimum degree of THV oversizing, as the expansion of the THV within the pre-existing ring is limited by the oval ring’s shorter diameter due to the rigidity of the annuloplasty ring material13,14. Several case reports have addressed this shortcoming by advocating closure of the residual leakage with plugs and occluders, or sizing strategies, respectively, which inevitably results in downsizing the implanted THV10,15-17.
To address this issue from a different angle, we developed an expandable surgical ring that can adjust to THV expansion and may thus render the TMVIR procedure safer in patients with recurrent MR. Specific features of our device are: 1) a changeable shape (oval towards round), 2) expandability, and 3) secure anchoring of the THV within the annuloplasty ring by introducing defined breaking points into the ring, and adding a non-tear, circular stainless steel cord into the ring that allows adequate THV oversizing and secure anchoring.
Methods
RING DESIGN
To assure expandability, a rigid, oval, saddle-shaped, three-dimensional annuloplasty ring (Profile 3D®; Medtronic, Minneapolis, MN, USA) was broken into four pieces at four precisely defined locations. The four segments were then reconnected with heat-shrinkable, semi-rigid tubing, securing the original three-dimensional shape of the ring (Figure 1). Then, we added a thin (0.8 mm diameter), non-tear stainless steel, circular cord (“limiting cord”) of defined length into the ring’s sewing cuff. In preliminary testing, 0.8 mm was the minimum wire diameter that resisted maximum balloon pressure without stretching or ruptures of the limiting cord. The length of this cord was adapted to the perimeter of the THV size to optimise the THV’s expansion and correct anchoring after deployment (Figure 1A-Figure 1D).
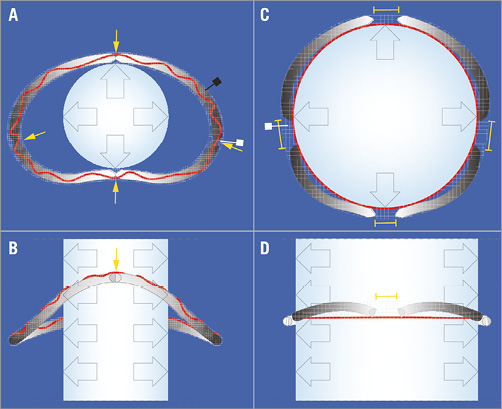
Figure 1. New annuloplasty ring. Transverse and coronal plane of the annuloplasty ring before (A, B) and after (C, D) balloon expansion. The yellow arrows in panel A indicate the four predetermined breaking points, the black square indicates a ring’s segment. The red line in panels A & B represents the unexpanded “limiting cord” within the sewing cuff. The yellow brackets in panels C & D indicate the gaps between the separated ring fragments after expansion of the annuloplasty ring; the red line in panels C & D represents the expanded “limiting cord” under tension. White squares in A & C: sewing cuff of the ring.
RING AND THV IMPLANTATION
We implanted our modified annuloplasty ring into a freshly explanted and preserved (Custodiol® HTK solution; Dr. F. Köhler Chemie GmbH, Bensheim, Germany) cadaver heart of a 25 kg pig (Figure 2). After implantation, the superior caval vein, inferior caval vein, and pulmonary veins, as well as the pulmonary artery and aorta, were occluded and the heart was pressurised by a cannula in the ascending aorta using physiological saline solution. A 23 mm THV (SAPIEN XT; Edwards Lifesciences, Irvine, CA, USA) was introduced transapically over an extra stiff guidewire (Amplatz® Extra-Stiff guidewire; Cook Medical, Bloomington, IN, USA). Correct positioning of the THV within the annuloplasty ring was confirmed by fluoroscopy (Veradius mobile C-arm; Philips Medical Systems, Eindhoven, The Netherlands) (Figure 3A-Figure 3E, Moving image 1). The crimped valve within the annulus was deployed stepwise and conformational changes were recorded simultaneously (Figure 3F, Moving image 2, Moving image 3). Lastly, the dilation balloon of the deployment catheter was expanded to a maximum extent in order to demonstrate the stability of the non-tear “limiting cord” (Figure 4A-Figure 4D).
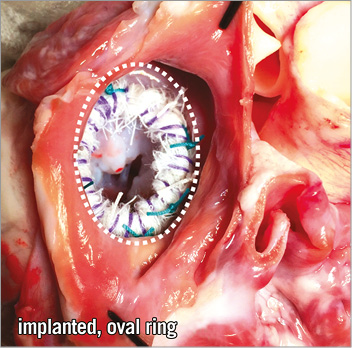
Figure 2. Implanted oval-shaped annuloplasty ring in the mitral valve annulus. Close-up picture of the opened left atrium of the excised pig heart showing the implanted new annuloplasty ring before THV deployment.
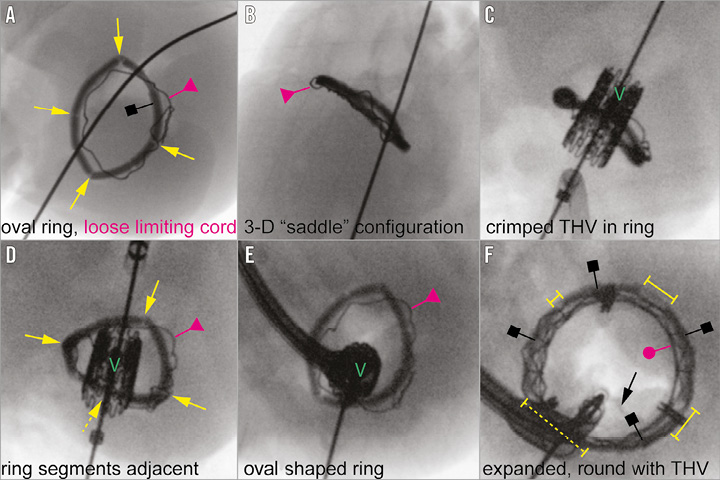
Figure 3. Fluoroscopic images showing the implantation procedure of the SAPIEN XT 23 mm THV within our modified annuloplasty ring. A transversal view of the annuloplasty ring with predefined breaking points (yellow arrows) and unexpanded limiting cord (red triangle; A & D). Panels B & C show in coronal view the THV being introduced into the annuloplasty ring (red triangle: loose limiting cord; “V”: THV). Panels D & E show the THV before balloon dilation, and panel F after dilation (yellow brackets: separated ring fragments; red dot: expanded limiting cord under tension after balloon dilation and THV implantation within the ring). Black squares in A & F: ring segments.
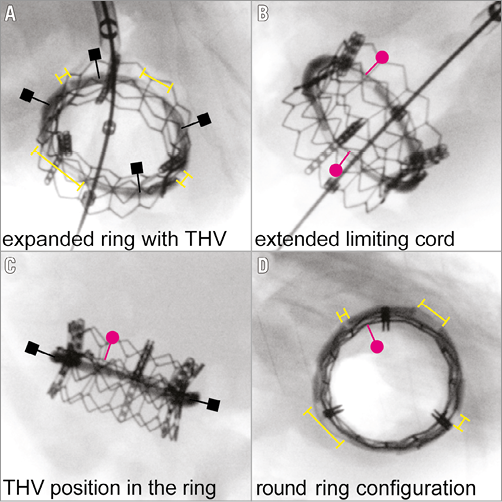
Figure 4. Implanted THV after overexpansion. Panels show the substantially enlarged gaps (yellow brackets in A & D) between the separated four ring segments (black squares in A & B). Note the maximally stretched non-tear steel limiting cord (red dots in B-D), limiting further expansion of the implanted THV.
Results
Before THV deployment our annuloplasty ring appeared in correct, three-dimensional, oval shape (Figure 3A, Figure 3B, Moving image 1). The radiopaque properties of the ring material facilitated fluoroscopy imaging for correct positioning of the THV (Figure 3C). Figure 3D depicts the four connected segments, maintaining the correct shape of the original ring. Before dilatation, the limiting cord was loosely positioned within the ring’s cuff and appeared twisted because of its larger perimeter compared to the oval-shaped, yet non-dilated annuloplasty device (Figure 3A-Figure 3E). After dilation, the four ring segments spread apart, creating large gaps between them. The ring expanded in a circular fashion, only limited by the steel cord, which was stretched to its maximum. The ring therefore changed its configuration from an oval to a round shape and its shorter diameter from 14 to 23 mm (Figure 3F, Moving image 2, Moving image 3). This enabled a secure landing zone for THV anchoring. Figure 5A-Figure 5D show the perfused heart and the expansion and shape-changing properties of our modified mitral annuloplasty ring, resulting in a circular geometry after balloon dilation without any sign of incomplete circumferential apposition.
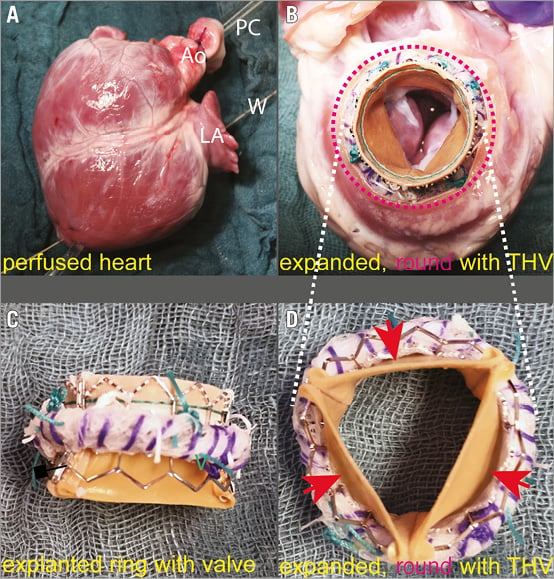
Figure 5. Pig heart before and after THV and ring expansion. A) Perfused heart during THV implantation (Ao: aorta; LA: left atrium; PC: perfusion cannula; W: extra stiff guidewire). B) Expanded and rounded (red dotted line) annuloplasty ring serving as an anchor for the SAPIEN XT 23 mm THV. C) Explanted ring with THV clearly demonstrates the transformed shape of the device from oval to round shape due to overextension and maximal stretching of the limitation cord. D) Confirmation of the absence of paravalvular leaks (red arrows) between the new annuloplasty ring and the SAPIEN XT 23 mm THV due to perfect alignment of the THV in relation to the ring.
Discussion
Recurrent MR after annuloplasty ring implantation may be treated by redo open heart surgery. However, especially patients suffering from recurrent MR due to ischaemic cardiomyopathy represent a high-risk surgical cohort. To address these issues, minimally invasive techniques using THV implantation in so-called valve-in-ring (VIR) procedures have been suggested9,18-20. In selected patient populations, several studies have shown the feasibility of this technique by demonstrating improvements in haemodynamic parameters and midterm functional status21-23. However, despite these promising results, technically challenging issues and various problems have arisen because of differences in geometry and material used for annuloplasty rings and THVs10,12-14. Thus, the search for the optimal types of ring and THV is still ongoing in order to find the ideal “THV-to-ring” match. For example, Wilbring et al implanted the 26 or 29 mm SAPIEN XT valve (Edwards Lifesciences) in different 28 and 30 mm annuloplasty rings in patients14. In sheep, Kondo et al examined THV annuloplasty ring configurations using the Melody® 26 mm valve (Medtronic) in four different commercially available annuloplasty rings (Carpentier-Edwards Physio; Edwards Lifesciences, partial ring, flexible ring and saddle ring)11. These studies reported the feasibility of VIR implantation using different commercially available rings with mostly reasonable results. Nevertheless, difficulties such as inappropriate oversizing of the THV were reported. These difficulties, related to the rigidness of the material used in the annuloplasty rings, resulted in more than mild remaining MR. In addition, unwanted configuration changes of the implanted THV from round to oval shape caused central or paravalvular leaks.
Recently, new transcatheter mitral valve devices with oval-shaped outer frames (e.g., Tendyne TMVR; Tendyne Holdings Inc., Roseville, MN, USA) which address the geometrical considerations better have been introduced in first-in-man studies in native mitral valves24. However, until these oval ring alternatives have been proved to be clinically applicable in VIR procedures, vascular occluders are suggested to seal paravalvular leaks between the circular THV and the oval annuloplasty ring, similar to those used to treat paravalvular leakage after THV implantation in failing surgical bioprostheses25. However, in patients who have undergone surgical annuloplasty with the aim of preserving the native and healthy mitral valve leaflets, these procedures do not meet the demands of a perfect match between the THV stent frame and the original annuloplasty ring if recurrent MR occurs and a VIR procedure is planned. Since THV confirmations cannot be changed, as they are implanted and dilated by a high-pressure balloon and are doomed to a round shape, the materials and/or configuration of annuloplasty rings will need to be changed13.
Actual, commercially available annuloplasty rings consist of various rigid, flexible, or formable materials in which THV implantation may fail due to suboptimal THV frame expansion or downsizing10,11,13,15,16. Therefore, we developed a universal concept to meet the demand for an expandable and shape-changing new annuloplasty device, which fulfils the requirements of the perfect THV-receiving device. These requirements are: 1) expandability to permit adequate oversizing of the THV, 2) change in configuration from “oval” to “round” to prevent central or paravalvular leakage, and 3) providing a stable boundary for secure anchoring of the THV. Our ring consisted of an oval, saddle-shaped, three-dimensional, rigid core that perfectly fitted in the mitral annulus when being surgically implanted. We introduced four predefined breaking points, which allowed the ring to separate into four independent segments, enabling the expansion and transition from oval to round. These features prevented paravalvular and central leakage and allowed the correct sizing of the THV. Within the sewing cuff of our annuloplasty ring, we provided a thin, non-tear stainless steel, circular cord (“limiting cord”) of a defined length that helped to anchor a THV that has a larger diameter than the annuloplasty ring itself. This allowed not only adequate oversizing, but also secure anchoring of the THV within the ring.
Our ring manipulation may be applied to various other ring shapes and materials from different manufacturers, although this remains to be tested. To explore its functionality, we implanted our newly designed annuloplasty ring into an isolated, perfused pig heart. Fluoroscopic evaluation of THV implantation with an Edwards SAPIEN XT 23 mm valve in the transformable ring showed optimal results with no observable leaks and great oversizing capacities. We anticipate similar results with larger balloon-expandable valves. Furthermore, our ring may also adapt to different self-expanding devices after perimeter-controlled predilatation.
Conclusions
Thus, we developed a transformable annuloplasty ring which anticipates a subsequent THV implantation. As there is a tremendous demand for a new generation of annuloplasty rings in the emerging field of VIR treatment for high-risk patients with recurrent ischaemic MR, our novel ring concept may help to stimulate further design modifications and device development. Furthermore, a similar mechanism could enable expansion of the stent internal diameter of smaller surgical mitral valves in VIV procedures, so that larger THV devices might be implanted. As known from the VIVID registry, small surgical valves in the mitral position (label 25 and below) are associated with a substantial risk of elevated gradients post VIV implantation, so a similar expansion mechanism to ours may be beneficial in valve prostheses as well. In these small annuli and valves, special attention should be paid to the enhanced risk of annular or myocardial rupture.
It should be noted that our new ring needs to be tested in further studies with respect to selection of the best material, fatigue testing, and functional evaluation, before it can be introduced in clinical practice.
Limitations
Mitral VIR procedures are associated with the risk of left ventricular outflow tract (LVOT) obstruction, as recently described26. As the conformability of the ring to the THV device and further expansion may, hypothetically, lead to more lateral displacement of the native mitral valve anterior leaflet, this might increase the LVOT obstruction risk even further. Blanke et al introduced a new “concept of the D-shaped annulus” assessment for transcatheter mitral valve implantation. It emphasises the need to respect thoroughly the LVOT dimension as well as the mitral valve to LVOT angulation, septal thickness, and left ventricular aortic angulation27. These measures can be identified on multidetector computed tomography scans and should be taken into consideration before VIR procedure as this could prevent overestimation of the THV size and therefore minimise the risk of LVOT obstruction. This concern should be explored in further feasibility testing, for example in an in vivo animal model. Also, future observational studies, such as the mitral VIVID registry, will have to elucidate the advantages of repositionable and fully retrievable devices such as the Lotus™ Valve System (Boston Scientific, Marlborough, MA, USA) or Direct Flow Medical® (Direct Flow Medical, Santa Rosa, CA, USA) prosthesis for VIR implantation, especially with regard to mitral regurgitation and LVOT obstruction compared to balloon-expandable valves.
A further concern might be that creating the ring’s predefined breaking points could weaken the annuloplasty ring and lead to spontaneous “fractures” during cardiac contraction and thus to a higher risk of annuloplasty failure. This has to be investigated further in in vitro (pulse duplicator) and in vivo (animal) studies. In order to guarantee safe function, material studies will be necessary to identify the best material for the ring’s core segments ensuring stability, and for the non-tear limiting cord ensuring the following features: tear-proof, defined length (circumference) and pliability. Also, fatigue testing of the newly developed expandable ring will have to be performed before it can be introduced into clinical practice.
Of course, as we proved our concept only in this single case, further studies have to be conducted with different THV and annuloplasty ring types. Bapat has described how three-dimensional rings (Edwards GeoForm ring, showing similar geometrical and material properties to the saddle-shaped Medtronic Profile® 3D ring) are unsuitable for VIR procedures using a SAPIEN XT, because of the high risk of paravalvular leak and SAPIEN valve deformation28.
In future studies, VIR procedures using different THV and annuloplasty ring types (1: complete rings; 2: partial rings, 3: saddle-shaped, three-dimensional rings; and 4: flexible rings), manipulated as described by us, should evaluate feasibility and optimal match between the devices in animal studies. The results will have to be compared with control cases using unchanged mitral ring types.
| Impact on daily practice Surgical ring annuloplasty using annuloplasty ring devices is currently the standard treatment option of choice for patients with ischaemic MR. As redo surgery in case of recurrent MR is associated with a high risk, minimally invasive transcatheter strategies are desirable and have already been tested – the so-called transcatheter mitral valve-in-ring (TMVIR) procedure. However, the oval configuration of the rigid annuloplasty rings often leads to suboptimal THV expansion, resulting in considerable paravalvular or central leakage. We therefore developed an annuloplasty ring with four predefined breaking points and an inner non-tear limiting cord, making a circular expansion and secure anchoring of a THV within the ring possible. This new annuloplasty ring might render TMVIR implantation safer and could replace high-risk redo surgery for the treatment of recurrent MR. |
Acknowledgements
We thank Dagmar Pasch for expert assistance with the illustration of Figure 1, and Sandra B. Haudek for helpful comments and revision of the manuscript.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplementary data
Moving image 1. THV in an intact, oval annuloplasty ring. The beginning of THV deployment within the still oval annuloplasty ring.
Moving image 2. Start of the THV expansion. Balloon expansion of the THV within the annuloplasty ring: conformational change from oval to round.
Moving image 3. THV deployment is completed. Enlarged gaps between the separated four ring segments with maximally stretched non-tear steel cord, limiting further expansion of the implanted THV.
Supplementary data
To read the full content of this article, please download the PDF.
Moving image 3. THV deployment is completed. Enlarged gaps between the separated four ring segments with maximally stretched non-tear steel cord, limiting further expansion of the implanted THV.
Moving image 1. THV in an intact, oval annuloplasty ring. The beginning of THV deployment within the still oval annuloplasty ring.
Moving image 2. Start of the THV expansion. Balloon expansion of the THV within the annuloplasty ring: conformational change from oval to round.

