The transcatheter direct mitral annuloplasty system (TDMA) is the closest approach to the “gold standard” of contemporary surgical annuloplasty techniques. TDMA reduces or eliminates regurgitation by effectively decreasing the septolateral dimension and increasing leaflet coaptation.
The DragonRing system (Valgen Medtech) is a novel TDMA system that consists of an anchor delivery system, a steerable sheath and a locker delivery system delivered by a transseptal approach (Figure 1A). The implantation is performed on the atrial side of the mitral valve annulus (MVA). The device system is composed of fully repositionable and retrievable anchors without polyester sleeves, a polymer-coated spacer, a radio-detectable contraction wire, and a locker (Figure 1B-Figure 1D). The device is easy to guide, and the guide sheaths and bending sheaths have a bending angle range of 0-270°. The indicator port and the scale can clearly show the real-time bending degree of the intracardiac device. The anchor, with a width of 2-3 mm, is designed to be further away from the coronary artery and coronary sinus, and the drill, with a length of 3-6 mm, is sufficient to provide a stronger and more stable anchoring force compared with earlier versions of TDMA systems, which make it suitable for patients with a posterior annulus length between 60 mm and 130 mm. The system is implanted in the beating heart on the posterior annulus under fluoroscopic and transoesophageal echocardiographic (TOE) guidance.
The first-in-human implantation of the DragonRing system was performed in a 77-year-old female with a 13-year history of atrial fibrillation who was admitted for progressive dyspnoea (New York Heart Association [NYHA] Class III-IV). TOE revealed an enlarged left atrium (43 mm), a dilated mitral annulus, inadequate leaflet coaptation, and subsequent severe (vena contracta=5×10 mm) atrial functional mitral regurgitation (AFMR) (Figure 1E, Moving image 1). The left ventricular ejection fraction (65%) and end-diastolic diameter (45 mm) were normal. The mitral valve (MV) annulus perimeter of 104.2 mm was measured by cardiac computed tomography (CT), and the distance between the anchors and the coronary artery was also evaluated after a simulated implantation (Figure 1F). TDMA was considered a reasonable therapeutic option. Under three-dimensional TOE guidance, the implanted catheter was navigated from the anterolateral commissure to the posteromedial commissure, to place 12 anchors at intervals of 8-12 mm and 9 spacers along the posterior annulus (Figure 1G, Moving image 2). Finally, the device was settled following adjustments of the contraction wire in real-time under digital subtraction angiography (DSA) and TOE guidance for optimal annular size (Figure 1H, Moving image 3). TOE showed mild mitral regurgitation (MR; 1+) (Figure 1I, Moving image 4). The postoperative CT-measured mitral annular area decreased from 8.15 to 5.23 cm2 (Figure 1J). The MV gradient was 2 mmHg and the results remained stable (mild MR) at the 6-month follow-up.
The present case demonstrated the feasibility of percutaneous mitral annular remodelling using a novel TDMA system with high device operability for the reduction of MR in the setting of AFMR. Future studies are required to confirm these initial results and define patient selection criteria for different transcatheter approaches to AFMR (Supplementary Figure 1).
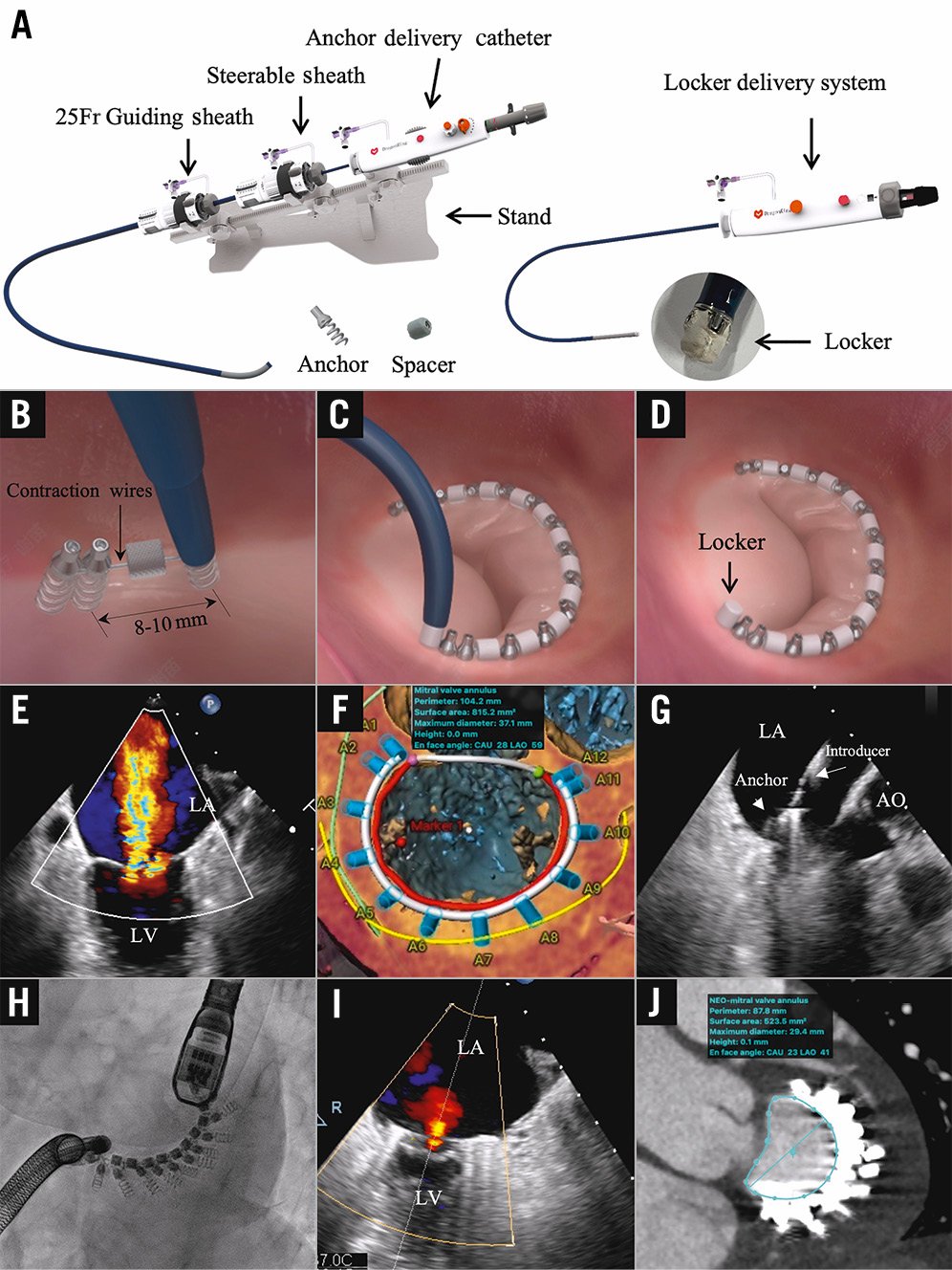
Figure 1. The DragonRing system and procedure. A) The DragonRing delivery system consists of an anchor delivery system, a steerable sheath and a locker delivery system with the DragonRing implants mounted on its distal end. B-D) The anchor or spacer is delivered and implanted, using the anchor delivery drive inside the implant catheter, into the annulus tissue. E) Severe mitral regurgitation in the two-dimensional transoesophageal echocardiogram (TOE). F) Computed tomography (CT) measurements and simulation of implants. G) Setting anchor by TOE guidance. H) Adjustments of the contraction wire under DSA. I) Postoperative TOE showed mild mitral regurgitation. J) Postoperative CT measurements. AO: aorta; CAU: caudal; DSA: digital subtraction angiography; LA: left atrium; LAO: left anterior oblique; LV: left ventricle
Conflict of interest statement
T-C. Zhang is an employee of Venus Medtech. Y. Feng and M. Chen are proctors/consultants for Venus Medtech. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.
Moving image 1. Severe mitral regurgitation in 2D transoesophageal echocardiographic cine (Figure 1E).
Moving image 2. Setting anchors by transoesophageal echocardiography guidance (Figure 1G).
Moving image 3. Adjustments of the contraction wire under DSA (Figure 1H).
Moving image 4. Postoperative transesophageal echocardiographic cine showed mild mitral regurgitation (Figure 1I).

