Mitral regurgitation (MR) is the most prevalent valvular heart disease in the United States1. A substantial proportion of MR patients are suboptimal surgical candidates due to their advanced age, comorbidities or frailty, and such patients may be better suited for transcatheter treatments. Transcatheter mitral valve replacement (TMVR) is emerging as an alternative for surgical high-risk MR patients2. However, the TMVR devices that are currently undergoing clinical testing have a high anatomical screen failure rate due to challenges with annulus size or left ventricular outflow tract (LVOT) obstruction34. The Saturn valve (InnovHeart) is a novel, low-profile TMVR bioprosthesis (available in 28 and 31 mm) that has a central valve which is mechanically connected to an annular ring. The aim of this study was to assess the procedural performance and long-term healing profile of this TMVR system in healthy preclinical models.
Procedural performance of the transseptal delivery system was tested using an acute porcine model (N=16), and the healing response was evaluated after transapical implantation in an ovine model (N=4). Moving image 1 shows a transseptal procedural animation. Valve performance and biocompatibility were assessed using fluoroscopy, echocardiography, and histology (Central illustration). All analyses were performed using Stata Version 12.1 (StataCorp). Continuous variables are expressed as mean±standard deviation.
For the acute study, 16 Yorkshire pigs (96.6±6.1 kg) that had undergone successful transseptal TMVR with the Saturn bioprosthesis were included in this analysis. Nine pigs were implanted with 28 mm valves and 31 mm valves were utilised in 7 pigs. The bioprostheses functioned well, with no paravalvular leak (PVL) and with mild or less intravalvular leak in all implants. The mean mitral gradients were 3.6±1.2 mmHg. There was no evidence of LVOT obstruction, with an LVOT gradient of 2.3±1.7 mmHg. Gross examination confirmed that all annular segments completely captured the mitral leaflets, embracing the entire subannular chordal structures. For the chronic study, 4 sheep (69.5±2.1 kg) underwent successful transapical implantation. Echocardiography post-deployment demonstrated excellent mitral pressure gradients (2.3±0.6 mmHg). None of the animals had PVL, and one animal had a trace intravalvular leak. All animals completed the study period of 150±7 days in good health, without any deterioration of cardiac function or clinical signs of heart failure. The haemodynamics of the bioprostheses remained excellent throughout the study with low mitral gradients (3.3±0.9 mmHg at 14 days, 3.1±0.9 mmHg at 30 days, 3.0±1.4 mmHg at 60 days, 3.0±0.0 mmHg at 90 days, 5.0±1.2 mmHg at 120 days, and 4.6±1.4 mmHg at 150 days). Paravalvular and intravalvular leak remained none to mild at all timepoints. Upon study completion, cardiac computed tomographies were performed, demonstrating excellent valve position without LVOT obstruction. Pathological assessment demonstrated an absence of significant calcification, thrombosis, and inflammatory response.
This bioprosthesis was designed with the goal to provide solutions for several of the challenges that are observed in TMVR. Of the patients with a treatable annulus size being considered for TMVR, up to 40% were previously reported as screen failures due to a perceived risk of LVOT obstruction. The Saturn valve has a low-profile design with a small footprint in the left ventricle (LV). In addition, the annular structure and the anterior connecting arm immobilise the anterior mitral leaflet, which prevents systolic anterior motion. In this preclinical series, we observed no cases of LVOT obstruction. This is despite the fact that porcine LVOTs tend to be small and the ventricular septum is somewhat hypertrophic. In addition to resizing and reshaping the annulus, the Saturn valve is designed to preserve the subvalvular apparatus. The annular structure surrounds the mitral chordae and is positioned in the LV just below the native mitral valve annulus. A self-expanding central valve is then mechanically connected to and expanded within this annular structure without further interaction with the native annulus or subvalvular apparatus. Preservation of the subvalvular apparatus maintains annular-papillary continuity, preventing distension of the ventricle which is well recognised to be associated with improved LV function, greater fractional change in LV end-systolic volume and survival in the surgical population5.
The main limitation of this study relates to the differences between a healthy animal model and human patients. In human MR patients, the mitral apparatus and the annulus can be diseased with a variety of pathological findings, including calcification, fragile or ruptured chordae, and degenerated or flail leaflets. None of these abnormalities can be sufficiently tested or accounted for in animal models.
In summary, this preclinical study evaluated the performance of the Saturn TMVR bioprosthesis. We demonstrated that 1) transseptal delivery of this bioprosthesis is feasible and reproducible and 2) that the valve has excellent haemodynamics with favourable biocompatibility over a 150-day follow-up period. These results support further investigation of this device in clinical studies.
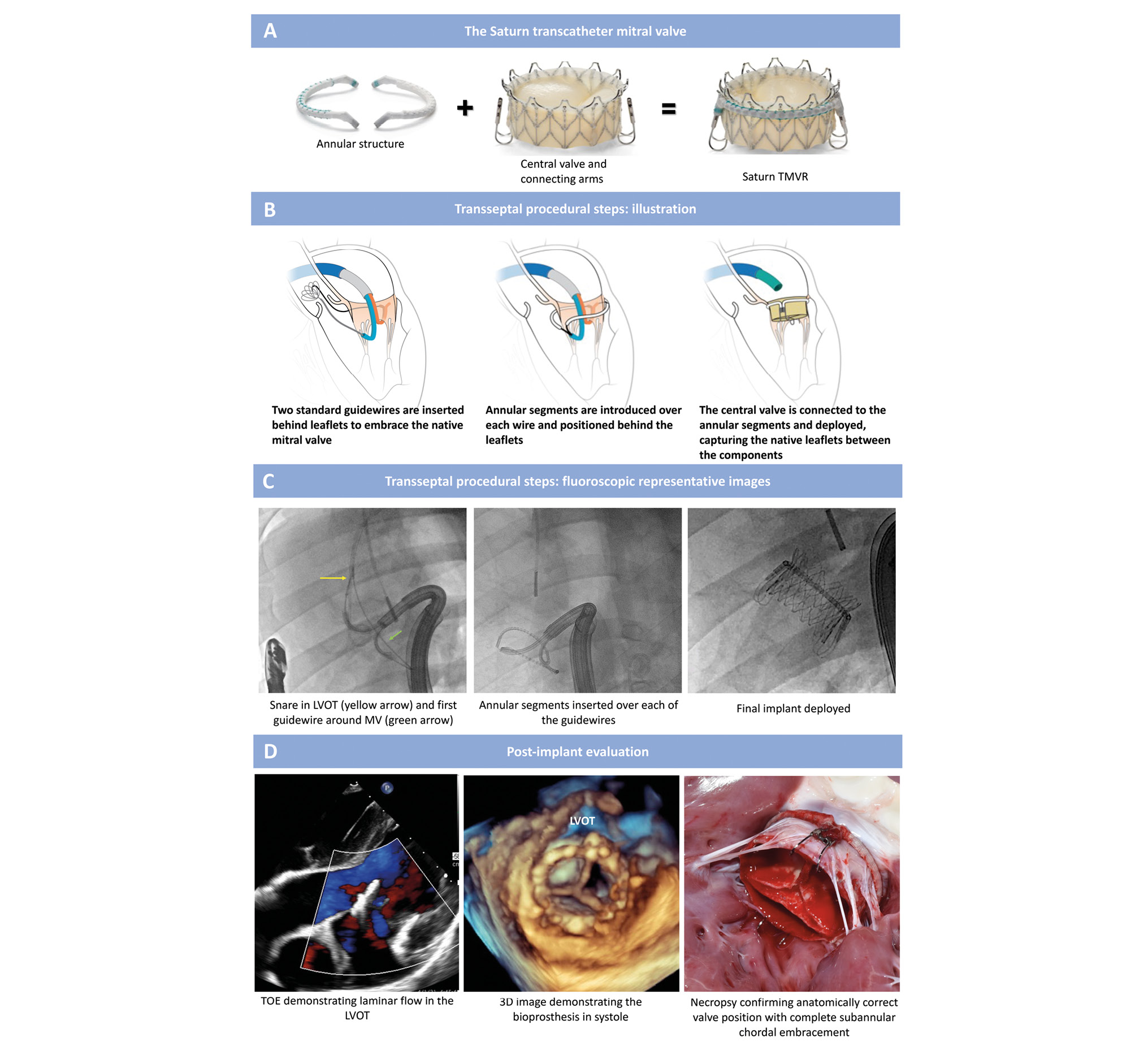
Central illustration. Saturn valve design and transseptal implantation. A) The Saturn TMVR has an annular structure that embraces the native mitral valve leaflets and mechanically connects to a central valve. The same prosthetic valve is compatible with both transapical and transseptal delivery systems. In the chronic study, the 28 mm size valve was deployed through a 39 Fr transapical port. In the acute study, the valve was deployed via a transseptal approach through a 29 Fr (inner diameter) steerable access sheath. B, C) There are three key procedural steps for transseptal valve delivery. After insertion of the guidewire delivery system through a steerable access sheath into the left atrium, the anterior and posterior arms are deflected at A2 and P2 behind the native mitral leaflets to enable the creation of medial and lateral loops (from P2 to A2) in the subannular groove. To complete the creation of the two loops, each of the guidewires is snared in the ascending aorta. Once both loops are formed, the medial and lateral annular segments are advanced over their respective guidewire rails. Lastly, the central valve is mechanically connected to the annular segments via the connecting arms to create a single prosthetic unit, and the central valve is deployed. D) Representative post-implant evaluation images demonstrating laminar flow in the LVOT by echocardiography and a well-seated valve with complete chordal embracement by necropsy. 3D: three-dimensional; LVOT: left ventricular outflow tract; MV: mitral valve; TOE: transoesophageal echocardiogram; TMVR: transcatheter mitral valve replacement
Funding
Funding for this study was provided by InnovHeart.
Conflict of interest statement
T. Vahl reports institutional funding to Columbia University Irving Medical Center from Boston Scientific, Edwards Lifesciences, JenaValve, and Medtronic; and he has received consulting fees from Abbott, Philips, InnovHeart, and 4C Medical. L. Ranard reports institutional funding to Columbia University Irving Medical Center from Boston Scientific; and consulting fees from InnovHeart, Philips, and 4C Medical. P. Denti reports speaker honoraria from Abbott and Edwards Lifesciences; and consulting fees from InnovHeart, Pi-Cardia, Approxima, Artiness, HVR, and Ventrimend. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.
Moving image 1. Procedural steps and workflow for the transseptal delivery of the Saturn TMVR device.

