Device description
Name and manufacturer: The Cardioband System; Valtech Cardio Ltd., Or Yehuda, Israel (Figure 1A).
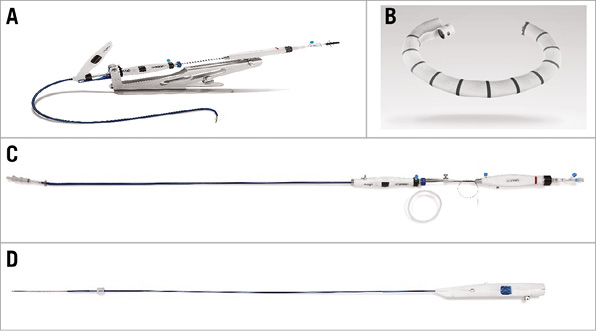
Figure 1. The Cardioband System. The Cardioband System (A) is composed of the implant (B), the delivery system (C) and the size adjustment tool (D).
Approval status: CE mark expected in 2015.
Implant technology: The Cardioband implant comprises three components: 1) a standard polyester fabric sleeve; 2) multiple stainless steel anchors; 3) an adjustment mechanism. The prosthesis is attached to the posterior annulus, trigone to trigone, via subsequent anchors. The adjustment mechanism is embedded inside the sleeve and after full deployment of the implant allows a homogeneous circumferential annular cinching (Figure 1B).
Delivery system: The Cardioband transfemoral delivery system consists of three components: 1) the implant delivery system features five degrees of control for continuous navigation across the mitral annulus (Figure 1C); 2) the anchor driver is used for anchor insertion with one-to-one torque transfer; 3) the size adjustment tool (Figure 1D), together with the adjustment mechanism inside the implant, provides a homogeneous stepwise cinching in which adequate remodelling is confirmed by echo on beating heart.
Delivery method: Transfemoral transseptal venous access.
Implant sizes: The Cardioband implant is available in six sizes to cover the different annulus sizes.
Procedural details
The procedure is stepwise, straightforward and reproducible, in which each step is reversible, resulting in an enhanced level of safety and control throughout. 1) Access: the device is introduced through the femoral vein to the right atrium and then through the fossa ovalis to the left atrium using a transseptal approach (Figure 2A). 2) Implant deployment: the implant delivery system is steered to reach any point on the mitral annulus. Subsequent anchors are deployed as the tip is navigated along the posterior annulus, as an annuloplasty ring would be sutured during an open heart procedure (Figure 2B, Figure 2C). 3) Implant adjustment, annular remodelling and MR reduction: after completion of implant deployment, the size adjustment tool (SAT) is introduced over a wire. By turning a knob, the implant is adjusted bi-directionally to reshape the annulus (Figure 2D), confirming MR reduction by echocardiography under beating heart.
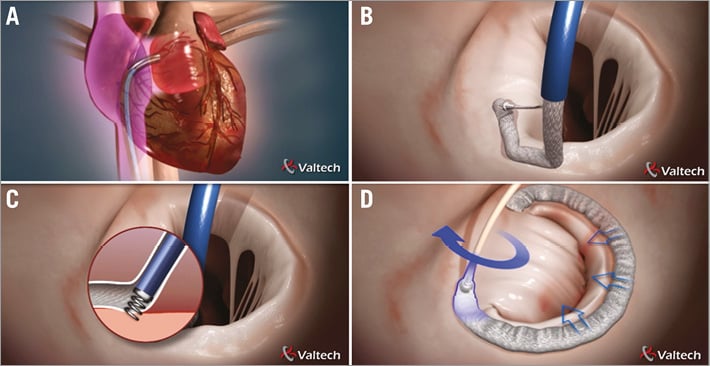
Figure 2. The Cardioband procedure. The procedure begins with transseptal access to the left atrium (A), then implant deployment (B), multiple anchor implantation (C), and size adjustment with mitral annulus remodelling (D).
Clinical data
The first-in-man (FIM) multicentre clinical study is currently ongoing, evaluating the feasibility, safety and performance of the Cardioband transcatheter adjustable mitral annuloplasty system in patients with functional mitral regurgitation (FMR). Between February 2013 and July 2015, 45 high-risk patients with significant FMR were enrolled at six sites in Europe. After a Heart Team evaluation, all patients were screened by echocardiography to assess eligibility.
Echocardiographic data were analysed by an independent core lab (Baylor Research Institute; Paul A. Grayburn, MD, USA). Mean age was 71±8 years; thirty-four patients were male (76%). Mean logistic EuroSCORE was 17±12%. At baseline, 87% of patients were in NYHA Class III-IV with mean EF of 32±11% (15%-59%). Device implantation was feasible in all patients (100%). MR reduction to ≤1+ was achieved in 84% of the patients (38/45) intra-procedure. After cinching of the device, remodelling of the mitral annulus with an average of 20% reduction of the septolateral diameter was observed (from 36±5 mm to 29±6 mm; p<0.01). Thirty-day mortality was 4.4% (adjudicated as unrelated to the device). At six-month follow-up, 82% of patients were in NYHA Class I-II (n=22) with significant improvement in quality of life (MLWHFQ from 38 to 18; p<0.05; n=21) and 86% of patients had MR ≤2+ (n=22). At 12-month follow-up, 94% of patients had MR ≤2+ (n=17), and 68% of patients were in NYHA Class I-II (n=18, p<0.05), confirming a positive trend in quality of life (MLWHFQ from 35 to 19; p<0.05; n=16). These results show the feasibility of transseptal direct annuloplasty with the Cardioband system, with a sustained safety profile, similar to other transcatheter mitral procedures. Effective reduction in MR severity is observed in most patients related to a significant septolateral dimension reduction. MR reduction is stable and consistent up to 12 months, with significant clinical benefit.
Ongoing studies
The Cardioband clinical study (NCT01841554) is currently ongoing.
Unique features
The Cardioband System features a unique segmental deployment that conforms easily to each patient’s specific annular geometry.
The venous access, combined with supra-annular fixation, as in surgery, and the stepwise procedure, which is reversible, guarantee an enhanced level of safety and control throughout the procedure1-3.
The implant enables significant reduction of annular dimensions, with preservation of the native anatomy.
Potential improvements
Cardioband, while reshaping and stabilising the mitral annulus, can be used in combination with other mitral valve transcatheter therapies, such as edge-to-edge repair or chordal replacement, to provide a surgical-like treatment to most types of mitral valve insufficiency4.
Conflict of interest statement
F. Maisano is a consultant for Abbott Vascular, Valtech Cardio, Medtronic, Edwards Lifesciences, St. Jude, and Apica, is co-founder of 4Tech, and receives royalties from Edwards Lifesciences. M. Taramasso has no conflicts of interest to declare.

