In this issue of EuroIntervention, two interesting case reports of patients undergoing transcatheter mitral valve-in-ring procedures with the Melody® transcatheter pulmonary valve (Medtronic, Minneapolis, MN, USA) are described1,2. The Melody prosthesis consists of a bovine jugular venous valve sutured into a platinum-iridium balloon-expandable stent. The company which produces the Melody valve recommends against its implantation in the aortic or mitral valve position, because of concerns regarding the durability of a jugular venous valve in the systemic circulation. As displayed by the current case reports, however, the Melody may be a viable option in patients lacking other suitable alternatives.
Several small series of Melody valve implantation in degenerated mitral valve (MV) bioprostheses have been reported3,4. Although the results have been favourable, these procedures have been limited to those patients with small tissue valves (i.e., 25 or 27 mm). The two case reports in the current issue of EuroIntervention appear to be the first descriptions of a transcatheter Melody valve-in-ring implantation in humans. Interestingly, both patients initially presented with a peri-annular leak post mitral repair surgery. Although the treatment algorithms were different, each of these authors utilised the Melody valve in a unique manner which may represent a new therapeutic option for transcatheter MV procedures.
The concept of beating heart mitral valve-in-ring implantation is not new and has been investigated in animal models for several years5. However, technical difficulties have limited its clinical application in humans. Mitral valve-in-ring procedures are more complex than mitral valve-in-valve implantation for several reasons. Firstly, the orifices of mitral annuloplasty rings are D-shaped and not spherical, resulting in incomplete sealing between the transcatheter valve and the surgically implanted ring. Small paravalvular leaks at both commissural regions are therefore encountered not infrequently during such procedures. More malleable balloon-inflation or self-expanding devices may be required in order to avoid such leaks. Secondly, mitral annuloplasty devices come in many different types and forms (i.e., flexible band vs. semi-rigid ring vs. rigid ring, complete vs. incomplete, flat vs. saddle-shaped), further complicating decision making when determining the appropriate transcatheter valve type and size. It is also important to note that the labelled size of a mitral annuloplasty ring is much larger than its actual anteroposterior (also known as “septolateral”) dimension, a measurement that is of the utmost importance when determining the appropriate size of the transcatheter valve. Thirdly, the retained native anterior MV leaflet may cause obstruction of the left ventricular outflow tract (LVOT) post device implantation. Preoperative determination of the length of the anterior MV leaflet as well as the LVOT diameter may help predict the occurrence of this complication. In addition, the depth of implantation may play a role, with more ventricular implants having a higher risk for this phenomenon. Finally, the native anterior MV leaflet may lead to impaired function of the transcatheter valve, an unusual complication that is analogous to that described during transcatheter aortic valve replacement6. In this situation, the retained anterior MV leaflet partially obstructs the outflow orifice of the transcatheter device, resulting in decreased valve closing forces and a large transvalvular leak. Such a complication is treated by emergent implantation of a second valve, slightly lower and within the first device.
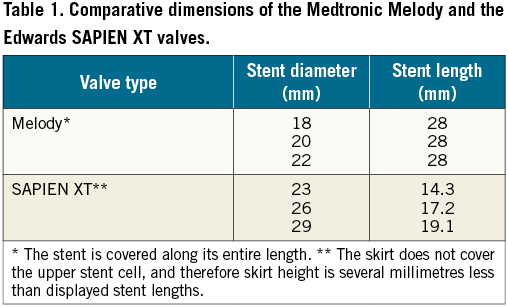
The two current case presentations demonstrate a potentially novel therapeutic approach to patients requiring a mitral valve-in-ring procedure. Until now, nearly all such procedures were performed with a balloon-expandable SAPIEN® or SAPIEN XT® valve (Edwards Lifesciences, Irvine, CA, USA). The SAPIEN valve requires sealing at the mitral annular level, since the skirt surrounding its leaflets is relatively short (Table 1). The Melody valve, however, is surrounded by a much longer stent that is covered throughout its length, which may allow for sealing to occur at the subvalvular (or, more appropriately, “subannular”) level, i.e., within the native mitral valve tissue rather than the annulus. Such a therapeutic approach appears not to have been attempted before in humans, and may have significant promise for future patients. It would eliminate the need for excessive dilation of a transcatheter valve in order to conform to the mitral annuloplasty ring, a manoeuvre that has been shown to result in significant transvalvular regurgitation7. The concept of subvalvular sealing may also lead to the development of new transcatheter MV replacement devices with a novel mechanism of action.
Maisano and colleagues1 postulated that they could use the covered stent design of the Melody valve in order to achieve sealing of the peri-annular MV leak at the subvalvular level, via a mini-thoracotomy transapical approach. Because of the large mitral annuloplasty size (i.e., 34 mm), they first inserted a 22 mm CP stent (NuMED, Inc., Hopkinton, NY, USA) as a landing zone for the Melody valve. In addition, the investigators had the creative idea of anchoring the Melody valve to the apex of the left ventricle with two GORE-TEX® sutures (Gore Medical, Flagstaff, AZ, USA), in order to prevent proximal embolisation of the device. Following deployment of the Melody valve within the CP stent, the opening of the peri-annular defect was still visible on echocardiography (Maisano’s Figure6C). However, flow was no longer present through this defect. In addition, only a small perivalvular/intra-annular leak was present, despite the large spaces between the Melody valve and the mitral annuloplasty ring (Maisano’s Figure6B). A proof of concept for the “subvalvular sealing” approach was therefore achieved.
The case from Kliger et al2 further enforces this proof of concept, but under different circumstances. Their patient presented with a peri-annular leak post MV repair, which was initially treated with an AMPLATZER™ Vascular Plug 4 (St. Jude Medical, Minneapolis, MN, USA). Although the decision to perform percutaneous closure of such a leak –rather than redo surgery– in a 49-year-old patient with near normal ejection fraction can be discussed critically, the authors achieved a satisfactory result under trying circumstances. After the initial closure attempts failed, the initial device was removed and an AMPLATZER™ Muscular VSD Occluder (St. Jude Medical) was placed, which resulted in acute severe transvalvular regurgitation. This was treated with emergent insertion of a Melody valve over a percutaneous arteriovenous rail, since a SAPIEN valve was not available at that time. A good seal was achieved between the 22 mm Melody valve and the 26 mm Physio mitral annuloplasty ring (Edwards Lifesciences) without evidence of paravalvular leakage. Given the experience of Maisano et al, one could retrospectively postulate that the sealing was achieved at the subvalvular level and that a Melody valve might have been sufficient to treat the original peri-annular leak, without the need for any AMPLATZER device closure.
The subvalvular apparatus as a new therapeutic option presents some challenges. Firstly, the subvalvular apparatus does not close in a purely circular fashion, as demonstrated by beating heart cardioscopy8. However, the MV leaflets may eventually fuse to the walls of a circular, covered device and thereby accomplish a complete seal. This is the most likely explanation for the disappearance of the new, anterolateral perivalvular leak during follow-up of the patient from Maisano et al (Maisano’s Figure 6C). Secondly, the risk of LVOT obstruction remains a concern when targeting the subvalvular apparatus, although this did not occur in the two case reports above. Further animal studies and clinical experience will be required in order to assess this potential risk.
One final issue should be addressed regarding the Melody valve. The small size of the prosthesis (i.e., 18, 20 and 22 mm) is a result of the normal diameter of the bovine jugular vein. The maximum theoretical orifice area achievable for a 22 mm valve is 3.8 cm2. Despite this somewhat limited orifice, low gradients have been demonstrated when the Melody valve is implanted in previous pulmonary and mitral bioprostheses3,9. In the above case reports, the mean transvalvular gradients were 4 and 5 mmHg, respectively. It appears that the thin, pliable leaflets and low-profile stent of the Melody valve lead to very good haemodynamic performance.
Although the Melody prosthesis is currently not recommended for insertion in the systemic circulation, the stent design and haemodynamic characteristics make it a viable option for patients without other alternatives. The “subvalvular sealing” proof of concept which has been demonstrated in the above case reports may also open the door to the development of novel transcatheter MV replacement devices. Although this form of therapy is still in its infancy, several interesting developments are sure to come.
Conflict of interest statement
M. Borger has received speaker’s honoraria from Edwards Lifesciences and St. Jude Medical, and is an SAB (Scientific Advisory Board) member for Sorin. S. Kodali is the principal investigator, PARTNER II (S3) Trial (Edwards Lifesciences), principal investigator, SENTINEL Trial (Claret Medical), and is an SAB member for Thubrikar Aortic Valve, Inc., and Meril Life Science.

