CASE SUMMARY
BACKGROUND: A 74-year-old male with symptomatic severe recurrent mitral regurgitation after surgical mitral valve repair and high surgical risk was referred for transcatheter mitral valve-in-ring implantation.
INVESTIGATION: Two- and three-dimensional transoesophageal echocardiography, fluoroscopy.
DIAGNOSIS: After transapical deployment of a 26 mm SAPIEN XT (Edwards Lifesciences, Irvine, CA, USA) in a mitral ring (Physio II ring, size 30; Edwards Lifesciences) and removal of the guidewire, severe para-ring mitral regurgitation originating at the level of the posterior commissure was detected.
MANAGEMENT: A 6 mm AMPLATZER™ septal occluder system (St. Jude Medical, St. Paul, MN, USA) was implanted at the level of the para-ring dehiscence with significant reduction in para-ring mitral regurgitation. Transthoracic echocardiography pre-discharge showed a mean transmitral gradient of 7 mmHg and minimal mitral regurgitation.
KEYWORDS: structural heart interventions, transcatheter mitral valve replacement, valve-in-valve, valvular heart disease
PRESENTATION OF THE CASE
A 74-year-old male with chronic atrial fibrillation, three-vessel coronary artery disease revascularised with coronary artery bypass grafting in 1997, and progressive symptomatic moderate to severe functional mitral regurgitation after inferior myocardial infarction in 2005 underwent transcatheter mitral valve repair in 2012 based on relatively high operative risk (logistic EuroSCORE of 13.9%). Associated comorbidities included chronic kidney disease and a prior cerebrovascular accident. Two MitraClips (Abbott Vascular, Santa Clara, CA, USA) were implanted at A2-P2 and A3-P3 levels of the mitral valve leaving the mitral valve without causing stenosis. At six-month follow-up, the patient presented with New York Heart Association (NYHA) functional Class III heart failure symptoms and recurrent severe mitral regurgitation was diagnosed on transthoracic colour Doppler echocardiography. The transoesophageal echocardiography (TEE) showed moderate to severe mitral regurgitation with two regurgitant jets between the two, well attached, MitraClips and between the device positioned at A3-P3 and the posterior commissure. The patient underwent surgical mitral valve repair and the direct surgical inspection of the mitral valve revealed a failed gripping of the MitraClip at the A3-P3 location caused by a severely calcified posterior mitral leaflet body and the lack of sufficient posterior leaflet tissue in that area. After removal of both clips, mitral annuloplasty was performed using a semi-rigid Physio II ring (model 5200, size 30L; Edwards Lifesciences, Irvine, CA, USA). Intraoperative TEE demonstrated a successful repair with no residual mitral regurgitation and a mean inflow gradient of 2.7 mmHg. However, at four-month follow-up, the patient was re-hospitalised with acute pulmonary oedema and remained in NYHA functional Class III afterwards, despite optimal medical treatment. Transthoracic echocardiography showed a non-dilated left ventricle with moderate reduction in ejection fraction (45%) and severe mitral valve regurgitation with restricted posterior mitral leaflet and broad vena contracta along the medial and posterior coaptation line. The mean gradient through the mitral valve was 3.8 mmHg and the estimated pulmonary pressure was 59 mmHg. The TEE showed the regurgitant jet impinging on the posterior annulus and small dehiscence at the level of the posterior commissure (Figure 1, Moving image 1-Moving image 3). Endocarditis, as a potential mechanism of recurrent mitral regurgitation, was excluded.
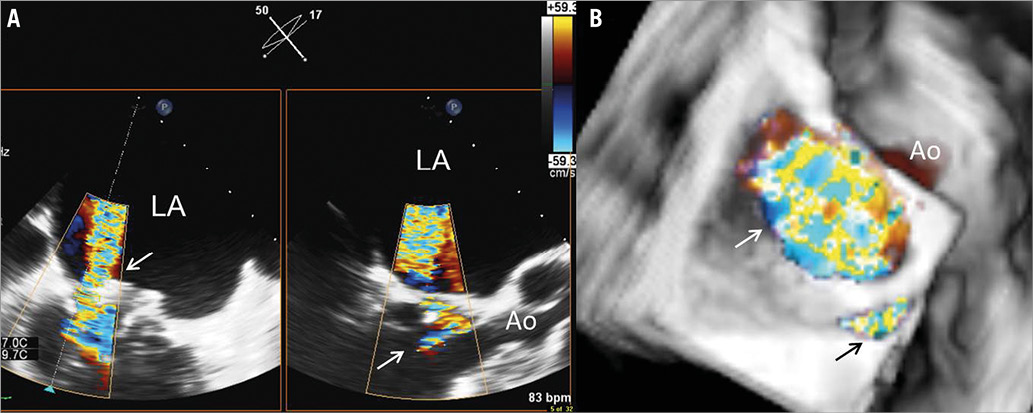
Figure 1. Three-dimensional transoesophageal echocardiography. A) Biplane view of the para-ring dehiscence with the regurgitant jet through the valve (arrow) in the right panel and the orthogonal view in the left panel showing the central regurgitant jet impinging on the ring. B) Three-dimensional reconstruction with an en face view of the ring. The central regurgitant jet through the valve (white arrow) can be observed together with the regurgitant jet through the para-ring dehiscence at the level of the posterior commissure (black arrow). Ao: aorta; LA: left atrium
Based on the associated comorbidities (chronic kidney disease and prior cerebrovascular accident), multiple cardiac surgical interventions (coronary artery bypass grafting and mitral valve repair) and the calculated logistic EuroSCORE of 24.8%, the Heart Team considered transcatheter mitral valve-in-ring the least invasive and most suitable treatment for this patient.
The procedure was performed under general anaesthesia assisted by fluoroscopic and two- and three-dimensional TEE guidance (EPIQ; Philips Medical Systems, Andover, MA, USA) equipped with a fully sampled matrix-array TEE transducer (X7-2t Live 3D-TEE transducer; Philips Medical Systems). Right femoral arterial access was obtained for continuous arterial pressure monitoring in the aorta and right femoral venous access for positioning of a temporary pacemaker wire using 7 Fr and 6 Fr sheaths, respectively. Through a left mini-thoracotomy, the left ventricular apex was exposed and purse-string suture was performed. A J guide was advanced through the mitral ring and positioned in the right inferior pulmonary vein. Next, the J guide was exchanged for an Amplatz 0.035”extra stiff wire (Cook Medical, Bloomington, IN, USA) followed by the introduction of a 24 Fr Ascendra sheath (Edwards Lifesciences) in the left ventricle. Subsequently, the mitral valve ring was ballooned under rapid right ventricular pacing with a 20×30 mm balloon (Ascendra Balloon Aortic Valvuloplasty Catheter; Edwards Lifesciences). Thereafter, the SAPIEN XT 26 mm (Edwards Lifesciences) was positioned across the mitral ring with 40% of the frame in the left atrium and 60% in the left ventricle and was deployed under rapid pacing (Figure 2). After removal of the Amplatz 0.035”extra stiff wire, colour Doppler TEE demonstrated the increase in mitral regurgitation originating from the dehiscence located at the posterior commissure (Figure 3, Moving image 4, Moving image 5).
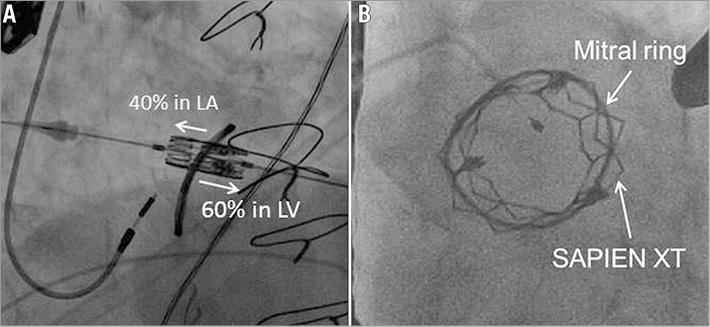
Figure 2. Fluoroscopy visualisation of the transcatheter mitral valve-in-ring procedure. A) Positioning of the 26 mm SAPIEN XT valve across the ring with 40% of the frame in the left atrium (LA) and 60% in the left ventricle (LV). B) After deployment under rapid right ventricular pacing, the left anterior oblique projection shows the transcatheter valve in the ring.
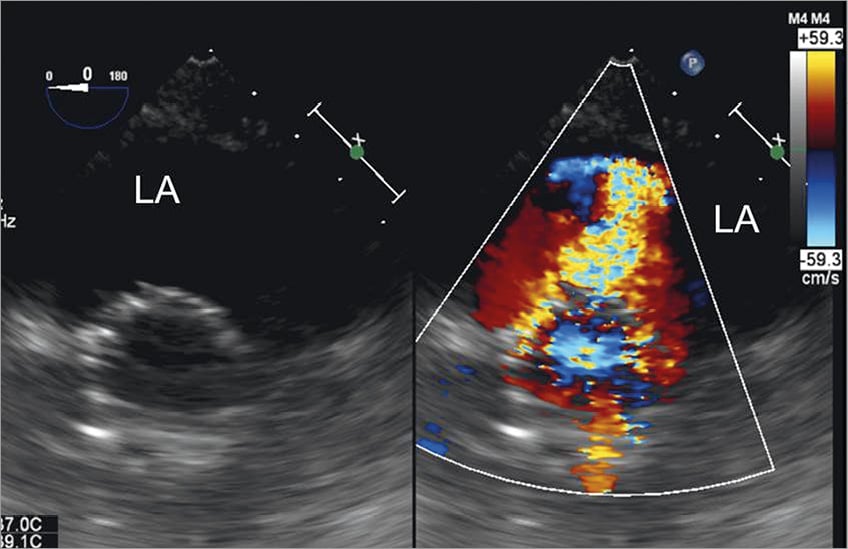
Figure 3. Transgastric view of the deployed transcatheter valve and evaluation of the results. Immediately after removal of the wire, transgastric transoesophageal colour Doppler echocardiography showed severe mitral regurgitation at the level of the posterior commissure.
How would I treat?
THE INVITED EXPERTS’ OPINION
The simplest, safest and fastest approach is the immediate closure of the leak with an AMPLATZER™ Vascular Plug III (AVP III; St. Jude Medical, St. Paul, MN, USA) and the use of the transapical Ascendra sheath (Edwards Lifesciences, Irvine, CA, USA) that is already in place as the access route. The considerations behind this decision are the following. 1) The valvular regurgitant jet has been successfully treated with the Edwards SAPIEN XT 26 (Edwards Lifesciences) using the valve-in-ring approach and the posteromedial paravalvular jet has significantly increased thereafter. If any doubt exists about the significance of the paravalvular jet, more elaborate echocardiographic measurements (3D vena contracta area) should be performed immediately, and additional measurements such as measuring v-wave in the left atrium (via the Ascendra sheath and the Edwards SAPIEN XT valve) could be carried out. However, we do not consider the latter necessary. 2) The patient has undergone repeated procedures and therefore a staged procedure involving additional general anaesthesia is unfavourable. In particular, a repeat transapical access route would be considerably more difficult, although not impossible. Moreover, an antegrade, transfemoral, and transseptal approach as a staged procedure does not offer any advantage and implies more limited steering capabilities. 3) All necessary staff and equipment are already present or available and the leak closure should be done within 30 minutes.
The mechanism behind the paravalvular regurgitant jet probably is that the Edwards Physio II ring had been sutured in soft not solid tissue in the posteromedial commissure, i.e., valve leaflet not fibrous annulus, which explains the tearing out of a few sutures (and valve tissue) only four months after ring implantation. At the time of Edwards SAPIEN XT valve-in-ring implantation and the resulting change into a more circular shape of the Physio II ring, two to three further sutures were torn out resulting in a significant increase in regurgitation. Yet despite this, an AVP III is considered safe when careful manipulation is performed.
The procedure in brief. Exact quantification of the short and long distance of the leak will be performed by colour Doppler imaging and the size of the AVP III will be selected accordingly. The leak will be crossed under fluoroscopy and 2D/3D TEE guidance via the Ascendra sheath with a standard 0.035’’ hydrophilic plastic-coated guidewire using a 5 Fr multipurpose diagnostic catheter for steering. The multipurpose catheter will be advanced into the left atrium and the plastic guidewire will be exchanged for an Amplatz 0.035’’ extra stiff wire (Cook Medical, Bloomington, IN, USA) followed by a 7 Fr or 9 Fr multipurpose guiding catheter as appropriate. The AVP III will be released by pushing the distal disc out of the sheath and pulling the sheath with the AVP III into the leak. Proper alignment of the long axis of the AVP III to the long axis of the leak can be well controlled by 3D echocardiography. The AVP III will be completely deployed by pulling back the sheath and sealing of the leak can be visualised immediately by colour Doppler imaging. After releasing the AVP III, removal of the Ascendra sheath and closure of the transapical access site will be performed as intended.
Conflict of interest statement
W. Schillinger has received proctor and lecture fees and travel expenses from St. Jude Medical, Edwards Lifesciences, and Abbott Vascular. R.S. von Bardeleben has received proctor and lecture fees and/or travel expenses from Philips Healthcare, Siemens and Abbott Vascular.
How would I treat?
THE INVITED EXPERTS’ OPINION
The authors describe a very complex case of a patient who has undergone a series of complex structural valvular interventions. These included the use of two MitraClips (Abbott Vascular, Santa Clara, CA, USA) to repair functional mitral regurgitation (MR), followed by surgical annuloplasty for recurrent MR. The patient then suffered from recurrent MR again and this was shown on echocardiography to be due to two mechanisms: malcoaptation of valve leaflets and dehiscence of the ring at the posterior commissure. The use of the Edwards SAPIEN XT 26 mm valve (Edwards Lifesciences, Irvine, CA, USA) adequately treated the central MR that was due to leaflet malcoaptation. However, the MR arising from the ring dehiscence remained and appears to be significant.
We propose the following treatment. First, the residual MR should be assessed for defect morphology and size. On the basis of the limited echocardiographic images provided, it would appear that the MR is at least 3+ in severity. As the MR from the ring dehiscence is worse at the end of the valve-in-valve procedure, we would proceed to treat the residual MR at the same time. This would take advantage of the existing access from the apex and offer a favourable trajectory for wiring the paravalvular leak.
An important consideration is the sizing of the paravalvular leak. The authors describe the leak as extending to 25% of the valve circumference, suggesting that more than one device may be necessary to close the defect. We anticipate that two devices may be necessary. A guide catheter (e.g., multipurpose) with angled Terumo Glidewire® (Terumo Corp., Tokyo, Japan) can be used to cross the paravalvular leak. The positioning of the catheters and wire will have to be carefully guided by TEE to ensure that they do not impinge on the newly deployed SAPIEN valve. Using the guide catheter, an AMPLATZER™ Muscular VSD plug (St. Jude Medical, St. Paul, MN, USA) can be delivered across the defect. The sizing will depend on TEE estimates but we suspect a 14 mm waist diameter device may be suitable initially. The device can be assessed to ensure stability, to evaluate residual MR and also to ensure that the SAPIEN valve is not impinged upon or affected. Depending on the residual MR, a second device, probably a smaller plug such as an AMPLATZER™ Vascular Plug 4 (St. Jude Medical) may be used to occlude the residual MR. Again sizing will depend on TEE guidance.
A catheter is then advanced via a wire next to the AMPLATZER Muscular VSD plug. Care should be taken not to dislodge the first device. The Vascular Plug 4 can then be positioned next to the first plug. Again, TEE should be used to assess stability, residual MR and effect on the SAPIEN valve. Once stability, efficacy and safety have been verified, the vascular plug can be deployed. Patients should remain anticoagulated throughout the procedure.
Conflict of interest statement
K.K. Yeo is a speaker for Abbott Vascular. The other authors have no conflicts of interest to declare.
How did I treat?
ACTUAL TREATMENT AND MANAGEMENT OF THE CASE
Severe para-ring mitral regurgitation left untreated may have important clinical prognostic implications and, therefore, conversion to surgical mitral valve replacement or transcatheter closure of the dehiscence was considered. Due to the high surgical risk, occlusion of the para-ring regurgitation using a transcatheter closure device was performed as bail-out manoeuvre. By aligning multiplanar reformation planes in the three-dimensional TEE images, an en face view parallel to the regurgitant orifice was reconstructed and in this view the size of the regurgitant orifice area was assessed (Figure 4, Moving image 6): 52 mm×39 mm. The left ventricular apex was partially closed and a 6 Fr sheath was reintroduced. With the support of a multipurpose catheter, a 0.038” J guide could be advanced through the para-ring regurgitation (Moving image 7) and a 0.014” BHW back-up wire was positioned next to it. By using a 6 Fr TorqVue™ 45º AMPLATZER™ delivery sheath, a 6 mm AMPLATZER™ septal occluder (St. Jude Medical, St. Paul, MN, USA) was implanted (Moving image 8): the first disc was deployed within the left atrium, just above the stent frame of the SAPIEN XT prosthesis, the waist was positioned at the level of the Physio II ring and the second disc just beneath the Physio II ring in the left ventricle (Figure 5). The regurgitant orifice was successfully occluded with residual mild para-ring regurgitation (Figure 6, Moving image 9, Moving image 10). The postoperative course was uneventful and the patient could be extubated within a few hours after the procedure. The transthoracic echocardiography performed before hospital discharge showed the stable position of the SAPIEN XT valve within the ring, with a mean gradient of 7 mmHg and minimal para-ring regurgitation.
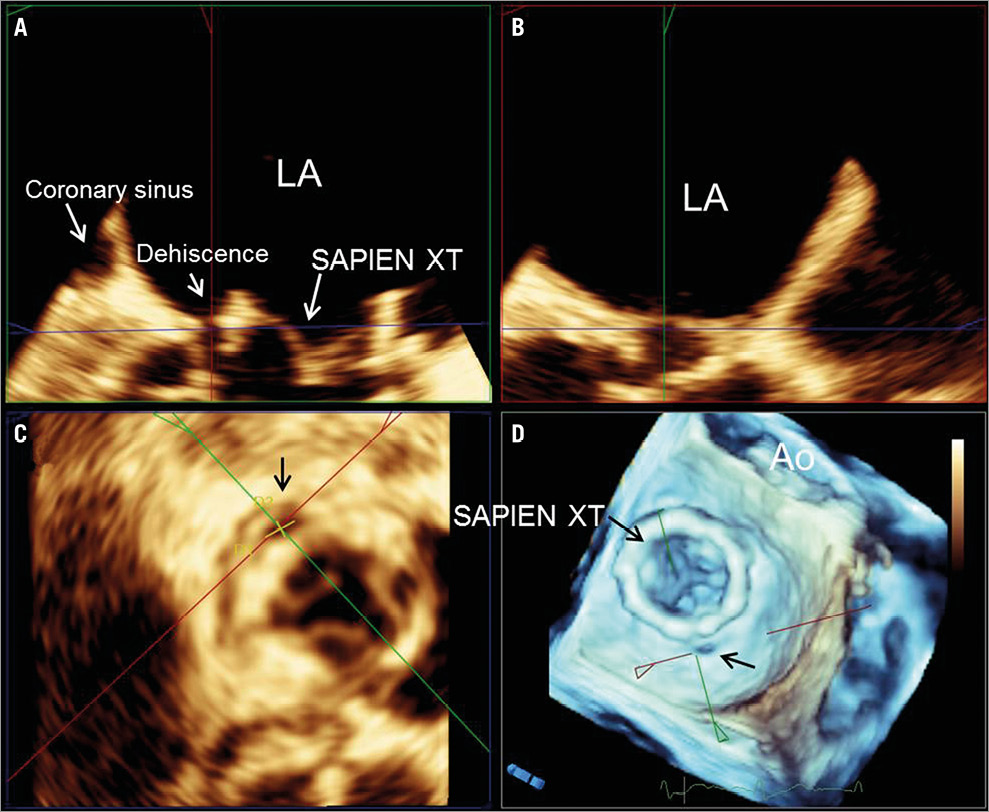
Figure 4. Three-dimensional multiplanar reformation planes across the para-ring dehiscence. By aligning multiplanar reformation planes across the dehiscence, the orthogonal long-axis views of the valve (A & B) and the transversal (C) views are shown. The en face view of the three-dimensional volume rendering with the crosshair through the dehiscence (D). The dimensions of the dehiscence can be obtained on the transversal view (C) in the three-dimensional transoesophageal echocardiography images.
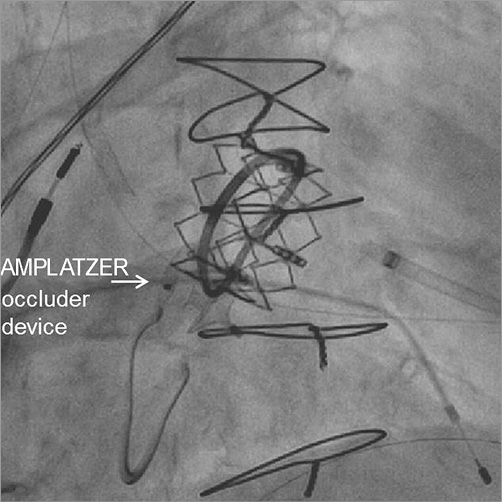
Figure 5. Final position of the AMPLATZER occluder device. To occlude the para-ring regurgitation, a 6 mm AMPLATZER septal occluder was implanted: the first disc was deployed within the left atrium, just above the stent frame of the SAPIEN XT prosthesis, the waist was positioned at the level of the Physio II ring and the second disc beneath the Physio II ring within the left ventricle.
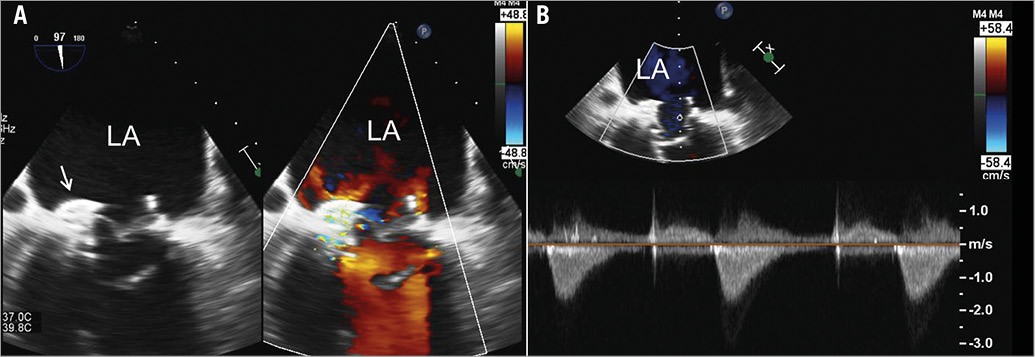
Figure 6. Evaluation of residual mitral regurgitation and haemodynamics of the valve-in-ring. A) On transoesophageal echocardiography the simultaneous visualisation of two-dimensional greyscale image and colour Doppler image permits the assessment of the position of the AMPLATZER occluder device and resolution of mitral regurgitation at the level of the para-ring dehiscence. B) Measurement of the transmitral gradient with continuous wave Doppler. At the end of the procedure, the regurgitant orifice was successfully occluded with residual mild para-ring regurgitation. The mean gradient was 7 mmHg.
Transcatheter valve-in-valve implantation has successfully become a feasible treatment option in patients with failed bioprostheses in aortic and mitral positions who are at high risk for redo surgery1. However, experience with transcatheter mitral valve implantation in a failed mitral valve ring annuloplasty is limited2-7. After a successful demonstration of the feasibility of implanting a 26 mm SAPIEN in a failed, 28 mm semi-rigid mitral Physio ring (Edwards Lifesciences, Irvine, CA, USA)2, this specific procedure has only been reported in case reports and small series3-7. Recently, Descoutures et al reported 17 high-risk patients with a failing surgical ring annuloplasty who underwent transcatheter mitral valve-in-ring implantation using a transvenous transseptal (n=8) or transapical approach (n=9)5. The procedural success rate was 88% and the 30-day survival was 82%, indicating that transcatheter valve-in-ring implantation is a feasible treatment option for these patients5. However, inappropriate deployment of the transcatheter valve within the ring (too high in the atrium or too low in the ventricle) and acute ring detachment after prosthesis deployment may result in insufficient reduction of mitral regurgitation5. Bail-out strategies such as implantation of a second transcatheter valve or conversion to open heart surgery may be needed. Transcatheter occlusion of paravalvular regurgitation in patients with dehiscent biological or mechanical mitral valve prostheses is a feasible and safe treatment in selected patients8. This is the first case report showing the feasibility of transcatheter occlusion of para-ring mitral regurgitation due to partial dehiscence of the ring after transcatheter valve-in-ring implantation.
Conclusion
Mitral ring dehiscence may occur after a valve-in-ring procedure causing significant mitral regurgitation. Dehiscence closure with an atrial septal defect closure device is a feasible bail-out manoeuvre to treat para-ring mitral regurgitation.
Funding
The Department of Cardiology received research grants from Biotronik, Medtronic, Boston Scientific, BMS Medical Imaging, Edwards Lifesciences, St. Jude Medical & GE Healthcare.
Conflict of interest statement
V. Delgado has received consulting fees from Medtronic and St. Jude Medical. The other authors have no conflicts of interest to declare.
Online data supplement
Moving image 1. Colour Doppler transoesophageal echocardiography. Mid-oesophageal view of the mitral annuloplasty at 0° showing the four-chamber views. The failure of coaptation between the anterior and posterior mitral leaflets leads to a severe regurgitation through the valve. The regurgitant jet impinges on the posterior annulus.
Moving image 2. Colour Doppler biplane view of the para-ring dehiscence. With three-dimensional transoesophageal echocardiography, the biplane view of the mitral valve permits simultaneous visualisation of the regurgitant jet through the para-ring dehiscence in two orthogonal views.
Moving image 3. Colour Doppler three-dimensional echocardiography. En face view of the mitral valve from the left atrial perspective. Colour Doppler data show the regurgitant jet through the valve and through the para-ring dehiscence located at the level of the posterior commissure.
Moving image 4. Simultaneous visualisation of two-dimensional greyscale and colour Doppler data of the mitral valve after deployment of the 26 mm SAPIEN XT valve. The normal function of the deployed transcatheter valve can be visualised with good coaptation of the leaflets and no regurgitation through it. However, the colour Doppler data show a significant regurgitation through the para-ring dehiscence.
Moving image 5. Simultaneous visualisation of two-dimensional greyscale and colour Doppler data of the mitral valve from a transgastric view. This view permits visualisation of the location and extent of the para-ring dehiscence. On colour Doppler data, 25% of the circumference of the valve is occupied by the regurgitant jet.
Moving image 6. Three-dimensional transoesophageal echocardiography. En face view of the mitral valve annuloplasty with the SAPIEN XT valve deployed. The para-ring dehiscence is located at the level of the posterior commissure.
Moving image 7. Three-dimensional transoesophageal echocardiography showing the guidewire through the para-ring dehiscence.
Moving image 8. Deployment of the AMPLATZER occluder device under three-dimensional transoesophageal echocardiography.
Moving image 9. Simultaneous visualisation of two-dimensional greyscale and colour Doppler data of the mitral valve from a transgastric view after deployment of the AMPLATZER occluder device. Note the significant reduction in para-ring regurgitation.
Moving image 10. Simultaneous visualisation of two-dimensional greyscale and colour Doppler data of the mitral valve after deployment of the AMPLATZER occluder device. Note the AMPLATZER occluder device with effective reduction of mitral regurgitation and the SAPIEN XT within the mitral ring with the native mitral valve leaflets beneath.
Supplementary data
To read the full content of this article, please download the PDF.
Moving image 1.
Moving image 10.
Moving image 2.
Moving image 3.
Moving image 4.
Moving image 5.
Moving image 6.
Moving image 7.
Moving image 8.
Moving image 9.

