Abstract
Aims: Recently, the feasibility of valve-in-valve procedures using current first-generation transcatheter heart valves (THV) in cases of structural valve degeneration has been reported as an alternative to conventional open repeat valve replacement. By design, certain biological valve xenografts carry a high risk of coronary ostia occlusion due to lateral displacement of leaflets after valve-in-valve procedures. In the present report we aimed to prove feasibility and safety of transapical valve-in-valve implantation of the JenaValve THV in two cases of degenerated Mitroflow bioprostheses.
Methods and results: We herein report two cases of successful transapical valve-in-valve procedures using a JenaValve THV implanted in Sorin Mitroflow bioprostheses for structural valve degeneration. Both patients were alive and in good clinical condition at 30 days from the procedure. However, increased transvalvular gradients were noted in both cases.
Conclusions: Transcatheter valve-in-valve implantation of a JenaValve THV is a valid alternative for patients with degenerated Mitroflow bioprostheses of sufficient size and in the presence of short distances to the coronary ostia who are too ill for conventional repeat open heart surgery. Increased pressure gradients have to be expected and weighed against the disadvantages of other treatment options when planning such a procedure.
Introduction
Transcatheter aortic valve implantation (TAVI) is an approved method for inoperable or high-risk patients with aortic stenosis unsuitable for surgical aortic valve replacement (AVR). Recently, the feasibility of valve-in-valve procedures using current first-generation transcatheter heart valves (THV) in cases of structural valve degeneration has been reported as an alternative to conventional open repeat valve replacement1,2. However, not all types of tissue valves are equally suited for such transcatheter approaches. A high risk of patient-prosthesis mismatch is known to be inherent in valve-in-valve implantation in degenerated aortic bioprostheses with nominal diameters below 23 mm3. Furthermore, by design, certain biological valve xenografts carry a high risk of coronary ostia occlusion due to lateral displacement of leaflets after valve-in-valve procedures. This has been reported in several cases of valve-in-valve procedures into Sorin Mitroflow bioprostheses4. In contrast to most other tissue valves, the Mitroflow valve features leaflets that are mounted on the outside of the valve stent resulting in lower distance to the coronary ostia especially in cases of narrow aortic root dimension.
We herein report two cases of transapical valve-in-valve procedures using a JenaValve (JenaValve Technology GmbH, Munich, Germany) THV implanted in Sorin Mitroflow® bioprotheses (Sorin S.p.A., Milan, Italy) for structural valve degeneration. Both patients presented with a pronounced operative risk profile precluding them from repeat open heart surgery by mutual agreement of the local interdisciplinary Heart Team consisting of cardiologists and cardiac surgeons. The JenaValve THV is available in three sizes (23, 25 and 27 mm) and has been CE marked for implantation in native aortic annuli ranging from 21 to 27 mm, both for calcified aortic stenosis as well as for non-calcified pure aortic regurgitation via the transapical access route. The prosthesis consists of a tri-leaflet porcine root valve mounted inside a nitinol stent featuring three positioning feelers which are meant for placement in the corresponding native aortic sinuses in the first step of the implantation technique. In the second step, the lower stent part is released from its housing, thereby engaging the native aortic valve leaflets (or as in this report the leaflets of the surgical bioprosthesis) by a clipping mechanism. Finally, the upper stent part is released and the device thereby fully deployed resulting in a supra-annular position. The advantage of the JenaValve THV in the present setting is that, by its clipping mechanism, leaflets of the surgical prostheses were firmly engaged, thereby eliminating the risk of coronary ostia obstruction.
CASE REPORTS
CASE 1
A 78-year-old male patient (body mass index [BMI] 29.7) was re-hospitalised with cardiac decompensation due to severe aortic regurgitation (AR). He presented with progressive dyspnoea equivalent to New York Heart Association (NYHA) functional Class III-IV and chest pain to the emergency room. There were no signs of myocardial ischaemia and the electrocardiogram (ECG) showed new onset of atrial fibrillation. Six years before, severe calcified aortic valve stenosis had been treated by implantation of a 25 mm Sorin Mitroflow bioprosthesis, and single-vessel coronary artery disease (CAD) with stenosis of the left coronary artery had been treated with a single left internal mammary artery (LIMA) bypass graft. Upon readmission, transoesophageal echocardiography (TOE) revealed severe regurgitation of the aortic valve substitute with transvalvular peak/mean gradients of 36/15 mmHg, mild mitral regurgitation and a preserved left ventricular ejection fraction. Coronary angiography showed an open LIMA graft and no significant progress of CAD. Further comorbidities included chronic obstructive pulmonary disease, chronic renal failure with a baseline creatinine of 1.9 mg/dl, atrial fibrillation and pulmonary hypertension with a right ventricular pressure of 55 mmHg.
By mutual agreement of the local Heart Team, an interventional approach was chosen due to the increased perioperative risk with a calculated logistic European System for Cardiac Operative Risk Evaluation I (EuroSCORE I) of 34.3% and a Society of Thoracic Surgeons Predicted Risk of Mortality (STS PROM) score of 8.1%. ECG gated contrast-enhanced computed tomography was performed and reconstructed using the 3mensio Medical Imaging software (3mensio Medical Imaging BV, Bilthoven, The Netherlands) for planning of the procedure, i.e., to assess annular and aortic root dimensions, to measure the height of the coronary take-off and to predict optimal C-arm angulation and the exact incision site. The scan confirmed a distance of only 5 mm between the Mitroflow sewing ring and the left ostium. Because of the inherent high risk for coronary ostium occlusion when using a conventional first-generation THV, we chose a 23 mm JenaValve prosthesis. Under general anaesthesia, in the hybrid operating room (OR), we first took a JL4 diagnostic catheter (Boston Scientific, Natick, MA, USA) and placed a guidewire into the left anterior descending artery for safety reasons (Figure 1A). Afterwards, access was gained via a skin incision of 4 cm and left anterior minithoracotomy through the fifth intercostal space. Subsequently, a 23 mm JenaValve THV was inserted and advanced past the degenerated Mitroflow prosthesis to a supravalvular position (Figure 1B, Figure 1C). After correct axial alignment (Figure 1D), the device was delivered stepwise, with development of the basal feelers in the first step (Figure 1E). Then, the device was retracted towards the annular level and checked for adequate placement of feelers in the corresponding valve leaflets (Figure 1F). After confirming correct seating in an alternative C-arm angulation, the clipping mechanism was actuated (Figure 1G) and in a final step the deployment was finalised by release of the upper stent part (Figure 1H). The whole implantation procedure was performed in beating-heart technique without the need for rapid ventricular pacing. Procedure and fluoroscopy times were 110 and 11.1 minutes, respectively, and a total of 140 ml of contrast agent was used. Aortic root angiography showed an adequate position of the valve without any valvular or paravalvular leakage (Figure 1I) and uncompromised coronary run-off. The complete angiographic sequence is provided in the online supplement (Moving image 1). Transoesophageal echocardiography confirmed good valve function with peak/mean transvalvular pressure gradients of 40/23 mmHg. The patient was extubated in the hybrid OR and transferred to the intensive care unit. The further postoperative course was uneventful except for mild pneumonia necessitating antibiotic treatment. Immediately prior to discharge, transthoracic echocardiography (TTE) confirmed adequate valve function with peak/mean pressure gradients of 52/22 mmHg and no paravalvular leakage (PVL). At 30 days of follow-up, the patient was doing fine with improved clinical symptoms of NYHA Class I-II.
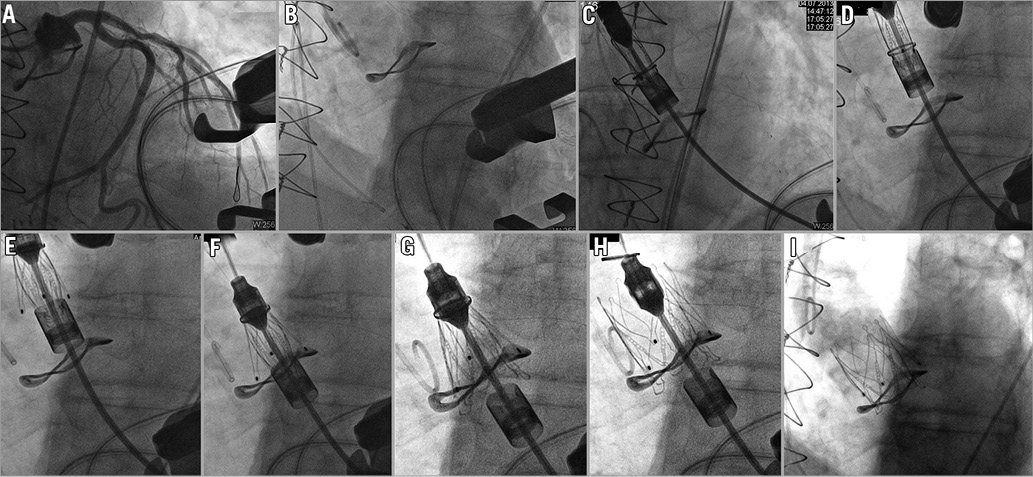
Figure 1. Angiographic sequence of a 23 mm JenaValve THV implanted into a degenerated 25 mm Sorin Mitroflow bioprosthesis via a minimally invasive transapical approach. A) Placement of a coronary guidewire in the left coronary artery for additional safety and performance of a coronary angiography. B) Aortic root angiography confirmed adequateness of predicted C-arm angulation with a perpendicular view on the Mitroflow sewing ring. C) Crimped JenaValve THV is inserted into the left ventricle and advanced into the ascending aorta. D) Axial alignment of the JenaValve THV. E) First delivery step: release of basal stent feelers. F) Retraction of JenaValve THV into the aortic root with positioning of each stent feeler in the corresponding prosthetic sinus. G) Second delivery step: release of the clip mechanism. H) Third delivery step: final deployment by release of the distal stent part. I) Final aortic root angiography documenting adequate valve function without paravalvular leakage and with preserved coronary perfusion.
CASE 2
A 79-year-old male patient (BMI 28.7) was re-hospitalised with cardiac decompensation due to severe AR. He presented with progressive dyspnoea equivalent to NYHA functional Class IV and severe peripheral oedema to the emergency room. Seven years before, severe calcified aortic valve stenosis had been treated by implantation of a 25 mm Sorin Mitroflow bioprosthesis, and single-vessel coronary artery disease with restenosis of the left coronary artery, after angioplasty and bare metal stent implantation in 2001, had been treated with a single LIMA bypass graft. TOE revealed severe regurgitation of the aortic valve prosthesis, a relative stenosis with a peak velocity of 4 m/s, transvalvular gradient of 15/8 mmHg, trivial mitral regurgitation and a preserved left ventricular ejection fraction. Coronary angiography showed an open LIMA graft and no significant progress of CAD. Further comorbidities included chronic obstructive pulmonary disease, peripheral artery disease, gastro-intestinal bleeding, renal failure and status post prostate cancer.
An interventional approach was chosen due to the increased perioperative risk with a calculated logistic EuroSCORE I of 33.2% and an STS PROM score of 6.1%. Because of a similar risk profile to the first case with a measured 6 mm distance between the Mitroflow sewing ring and left main stem, the decision was made for a 23 mm JenaValve THV. After an uneventful implantation the angiogram showed a not fully expanded valve stent, and TOE revealed an increased transvalvular pressure gradient requiring post-dilatation. Procedure and fluoroscopy times were 75 and 6.8 minutes, respectively, and a total of 65 ml of contrast agent was used. The patient was extubated in the hybrid OR and transferred to the intensive care unit. The further postoperative course was uneventful and the patient was discharged after seven days. Immediately prior to discharge, TTE confirmed adequate valve function with peak/mean pressure gradients of 38/22 mmHg and no paravalvular or valvular leakage.
Discussion
Potential complications of a valve-in-valve procedure in a Mitroflow prosthesis arise from the design of the valve. The leaflet tissue is mounted externally over the stent as opposed to internally, as is generally the case with other commercially available surgical prostheses. Furthermore, the height of the valve is relatively tall and the sewing cuff is relatively shallow. All of these features increase the risk of coronary ostia obstruction during a valve-in-valve procedure when using conventional first-generation THV. In the described cases, valve-in-valve procedures were performed in degenerated 25 mm Mitroflow prostheses which had an internal diameter (ID) of 21 mm and a height of 15 mm. Technically, valve-in-valve procedures in these specific cases could also have been performed using a 23 mm Edwards SAPIEN (Edwards Lifesciences, Irvine, CA, USA), a 26 mm Medtronic CoreValve (Medtronic, Minneapolis, MN, USA), or a 23 mm St. Jude Medical Portico THV (St. Jude Medical, St Paul, MN, USA). However, due to reports on cases of coronary obstruction following transcatheter valve implantation4-6, the decision was made for a 23 mm JenaValve THV. Another possible alternative would have been use of a Medtronic Engager THV. This valve has a somewhat similar concept to the JenaValve in that it also by design engages the native (or prosthetic) valve leaflets.
The 23 mm JenaValve THV has an unrestricted ID of 23 mm and a stent height of 30 mm and, since it is approved for implantation in aortic annuli ranging from 21 to 23 mm in diameter, it was chosen in the described cases. One advantage of the JenaValve is the clipping mechanism, which allows the patient’s native leaflets to be clipped to the valve stent, thereby eliminating the risk of coronary artery occlusion (Figure 2). However, the JenaValve was not designed for valve-in-valve procedures, and incomplete stent expansion in the presented cases has probably contributed to increased mean transvalvular pressure gradients. This seems even more plausible, since implantation of the JenaValve in the native aortic valve has been reported to yield favourable haemodynamic results. In the multicentre CE mark trial of the device, mean transvalvular gradients were 10.0±7.2 mmHg post-procedurally. Also, the unrestricted outer diameter of a 23 mm JenaValve is 28 mm according to the manufacturer’s specifications. In the presented cases, stent expansion was naturally limited to the true ID of 21 mm of the Mitroflow prosthesis. These technical limitations were discussed within the Heart Team preceding the procedures. However, the described approach was deemed the safest option for the patients, elevated residual gradients were to be expected, and this result had to be accepted in these elderly, comorbid patients.
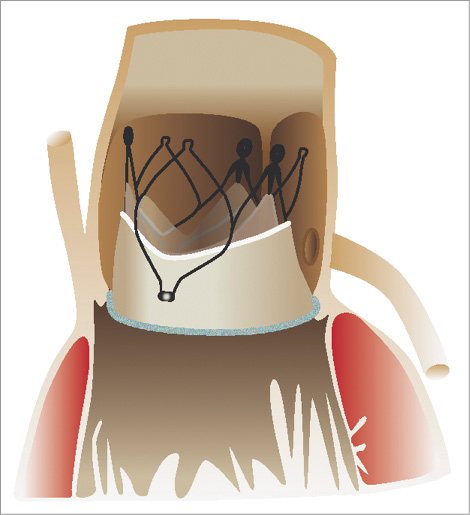
Figure 2. Schematic drawing of a JenaValve THV seated inside a Sorin Mitroflow bioprosthesis. By engaging the prosthetic leaflets the risk of coronary obstruction is eliminated.
Conclusion
Transcatheter valve-in-valve implantation of a JenaValve THV is a valid alternative for patients with degenerated Mitroflow bioprostheses of sufficient size and in the presence of short distances to the coronary ostia who are too ill for conventional repeat open heart surgery. Increased pressure gradients have to be expected and weighed against the disadvantages of other treatment options when planning such a procedure.
| Impact on daily practice The present report of transapical aortic valve-in-valve implantation using a JenaValve THV in two cases of degenerated Mitroflow bioprostheses demonstrated the safety and feasibility of the procedure. This technique may add to the armamentarium of physicians when dealing with cases of aortic bioprosthetic degeneration in patients at high risk for repeat open surgical aortic valve replacement, especially in cases of degenerated Mitroflow prostheses and difficult aortic root anatomy. |
Conflict of interest statement
L. Conradi and M. Seiffert received lecture fees and travel compensation from JenaValve Technology GmbH. J. Schirmer is a proctor for JenaValve Technology GmbH. H. Treede is a proctor and advisor for JenaValve Technology GmbH. The other authors have no conflicts of interest to declare.
Online data supplement
Moving image 1. Angiographic sequence of a 23 mm JenaValve THV implanted into a degenerated 25 mm Sorin Mitroflow bioprosthesis via a minimally invasive transapical approach.
Supplementary data
To read the full content of this article, please download the PDF.

