
Abstract
Aims: The aim of this study was to report clinical outcomes of self-expanding transcatheter aortic valve implantation (TAVI) for failed small Mitroflow (MF) bioprostheses.
Methods and results: Between January 2013 and July 2016, 15 symptomatic patients (NYHA Class ≥III) with degenerated small MF (≤23 mm) underwent CoreValve (CV) or Evolut R (EvR) implantation due to high/prohibitive risk for surgical redo. The MF size was 19 or 21 mm (off-label in Europe) in eight patients. A “preventive” left main (LM) stenting was successfully performed in one patient. Early LM obstruction occurred in two cases requiring stenting. Late LM obstruction was observed in one subject. A significant correlation between virtual left transcatheter valve-to-coronary ostia (VTC) distance and left sinus of Valsalva (LSV) diameter was observed (R=0.652; p=0.012). However, only left VTC was significantly smaller in patients who experienced LM obstruction compared to those who did not (p=0.002). No cases of moderate/severe stenosis were observed in either on- or off-label procedures. No death or other major events occurred up to the one-year follow-up.
Conclusions: CV or EvR implantation for failed small MF has favourable early and midterm outcomes if a careful risk evaluation and preventive measures for coronary obstruction are adopted. Low gradients can be achieved regardless of MF size.
Abbreviations
CABG: coronary artery bypass graft
CPB: cardiopulmonary bypass
CT: computed tomography
CV: CoreValve
EOA: effective orifice area
EvR: Evolut R
ID: internal diameter
LAD: left anterior descending
LCH: left coronary height
LIMA: left internal mammary artery
LM: left main
LSV: left sinus of Valsalva
MF: Mitroflow
NYHA: New York Heart Association
RCH: right coronary height
RSV: right sinus of Valsalva
STJ: sinotubular junction
STS: Society of Thoracic Surgeons
SV: sinus of Valsalva
SVH: sinus of Valsalva height
TAVI: transcatheter aortic valve implantation
VARC-2: Valve Academic Research Consortium-2
ViV: valve-in-valve
VTC: virtual transcatheter valve-to-coronary ostia
Introduction
Transcatheter aortic valve implantation (TAVI) in degenerated surgical aortic bioprostheses is an emergent treatment option for patients at high or prohibitive surgical risk. However, valve-in-valve (ViV) procedures carry some safety and efficacy concerns, particularly related to the increased risk of coronary obstruction and post-procedural gradients1.
In the Global Valve-in-Valve International Data (VIVID) registry, patients with a Mitroflow (MF) valve (Sorin Group Inc. [now LivaNova], Milan, Italy) had a 30-day mortality rate which was more than double compared to those with other types of stented valve2. MF leaflets are mounted on the external side of the valve frame; this particular characteristic increases the risk of coronary obstruction during ViV procedure and could affect early survival. On the other hand, the risk of residual stenosis is particularly evident in patients with a small bioprosthesis (internal diameter [ID] <20 mm). This subgroup of patients has a lower one-year survival as compared to those with larger valves2. Moreover, manufacturing companies discourage TAVI in degenerated surgical bioprostheses with IDs smaller than those recommended (i.e., in Europe, CoreValve® [Medtronic, Minneapolis, MN, USA] 23 mm implantation in degenerated MF 19 and 21 mm is considered an off-label procedure).
Currently, data on TAVI for failed small MF are limited and reported in the context of registries or case series including patients with different surgical bioprostheses treated with different transcatheter aortic valves3-7.
Our aim was to report early and midterm outcomes of consecutive patients undergoing CoreValve (CV) or Evolut™ R (EvR; Medtronic) 23 mm implantation for failed small MF focusing on: i) risk evaluation, prevention and treatment of coronary obstruction; and ii) assessment of post-procedural gradients according to MF size (on-label vs. off-label procedures).
Methods
STUDY POPULATION
We retrospectively analysed all consecutive patients who underwent TAVI (CV or EvR 23 mm) for failed small MF bioprostheses at our institution between January 2013 and July 2016. Diagnosis of bioprosthesis degeneration had been made by transoesophageal echocardiography with assessment of aetiology (i.e., stenosis, regurgitation or combined)8 and predominant mechanism of MF failure9,10.
Briefly, bioprosthesis degeneration can occur due to leaflet or non-leaflet failure or both. Leaflet failure is the result of degeneration (wear and tear or calcification) or destruction (endocarditis). Non-leaflet failure is the result of pannus, thrombus or paravalvular leak9,10.
All cases were extensively discussed by a team of specialists (cardiac surgeons, interventional and clinical cardiologists, anaesthetists), who deemed patients at extreme or prohibitive risk for surgical redo because of comorbidity or frailty. All endpoints were defined according to the Valve Academic Research Consortium-2 (VARC-2) recommendations11. Follow-up visits were performed at 30 days and one year from the procedure.
COMPUTED TOMOGRAPHY EVALUATION
A retrospective analysis of ECG-gating contrast-enhanced multislice computed tomography (CT) was performed separately by two expert physicians and reviewed by a third reader for consensus when there was disagreement.
All quantitative parameters collected were reported in millimetres (mm) and included: i) right and left coronary height (RCH and LCH), defined as the perpendicular distance between the ring plane and the coronary ostia; ii) right and left sinus of Valsalva (RSV and LSV) diameters; iii) average sinus of Valsalva height (SVH); iv) average diameter of the sinotubular junction (STJ); and v) distance from the virtual transcatheter valve (ring at the level of the top of the posts and in a size of TAVI device to be implanted at that level [20 mm]) to coronary ostia (VTC distance, right and left)12,13. The qualitative parameters were: i) presence and patency of coronary bypass grafts; and ii) MF coaxiality, defined as the alignment between a virtual ring defined by the posts and the aortic root (central or versus right, left or non-coronary sinus).
MITROFLOW FEATURES
The MF has a silicone base ring, an acetyl homopolymer stent and bovine pericardial leaflets that are mounted on the external side of the valve frame.
Patients included in the study had MF sizing 19, 21 or 23 mm with an ID of 15.5, 17 or 19 mm, respectively. Therefore, all patients were considered to have a small bioprosthesis (ID <20 mm)2.
According to the Medtronic CE instructions for use (IFU)14, the smaller CV and EvR prostheses (23 mm) can be used for annulus sizes between 18 and 20 mm. Hence, in Europe, CV and EvR 23 mm are not recommended for failed MF ≤21 mm because of the risk of incomplete prosthesis expansion that may result in poor leaflet coaptation or redundant leaflets. Accordingly, we stratified our population into two groups, off-label (MF 19 and 21 mm) and on-label (MF 23 mm).
PROCEDURE AND DEFINITIONS
Details about the procedure have been reported previously9.
Since October 2014, the CoreValve 23 mm has been replaced by the new repositionable and recapturable device, Evolut R 23 mm.
For all procedures, a standby for cardiopulmonary bypass (CPB) was arranged in order to deal with potential complications quickly. Standby for CPB was defined as: i) arterial and venous vascular accesses prepared (5 Fr sheaths without cannula insertion); ii) CPB machine and operators ready in the catheterisation laboratory.
Implantation height was assessed as recently reported15.
The “tunnel” technique was performed in case of coronary occlusion, as previously described16.
The “chimney” technique, reported here for the first time, was a strategy to prevent coronary obstruction. It consisted of stenting the LM, with a large portion of the device floating in the ascending aorta, immediately before completing self-expanding valve deployment. The details are reported in Figure 1.
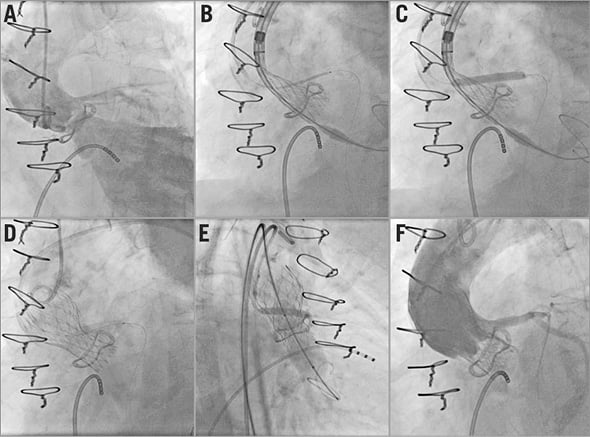
Figure 1. The “chimney” technique. Severe aortic regurgitation and left coronary dominance are evident at the baseline aortography (A). A MULTI-LINK VISION® stent (Abbott Vascular, Santa Clara, CA, USA) 4.5×28 mm is positioned in the left main through a guiding catheter 6 Fr EBU 3.0 and a BHW wire (B). When two thirds of the Evolut R 23 mm is released, the coronary stent is deployed in the left main with a large portion floating in the ascending aorta (C). Then, the self-expanding prosthesis is completely released (D). Post-dilation with an 18 mm Cristal balloon (Balt, Montmorency, France) and coronary stent inflation are simultaneously performed (“kissing balloon”) (E) with a good final result: patency of left main and lack of aortic regurgitation (F).
After the procedure, patients were treated with clopidogrel on top of aspirin for three months unless concomitant conditions required anticoagulation.
STATISTICAL ANALYSIS
Continuous variables were reported as mean±standard deviation or median (interquartile range [IQR]) and compared using the Student’s t-test or Mann-Whitney test, respectively. Categorical variables were reported as counts and percentages and compared using the chi-square test. Correlation between continuous variables was carried out using the Pearson test.
For all analyses, a two-sided alpha <0.05 was considered to be significant. All statistical analyses were performed using SPSS software, Version 21 (IBM Corp., Armonk, NY, USA).
Results
BASELINE CHARACTERISTICS
During the observation period, 15 patients with degenerated small MF bioprostheses underwent TAVI with CV or EvR 23 mm at our institution.
Baseline features of the study population are reported in Table 1. Seven patients (46.7%) were male and the mean age was 79±5 years. The patients were severely symptomatic (NYHA Class ≥III) and at high surgical risk with a median STS score and EuroSCORE II of 7% (IQR 5-12) and 18% (IQR 12-33), respectively.

The MF size was 19 or 21 mm in more than half of the population for whom the procedure was considered off-label (Table 1).
The aetiology of degeneration was stenosis in three patients, regurgitation in six cases and combined in the remaining six subjects. The predominant mechanism of failure was calcification in seven cases, wear and tear in six patients and previous endocarditis in the remaining two subjects (Table 2). Three patients had coronary bypass grafts (Table 1) patent at the CT analysis. CT data, available for all but one patient, are shown in Table 3.

Of note, a significant correlation between left VTC distance and LSV diameter was observed (R=0.652; p=0.012) (Figure 2).
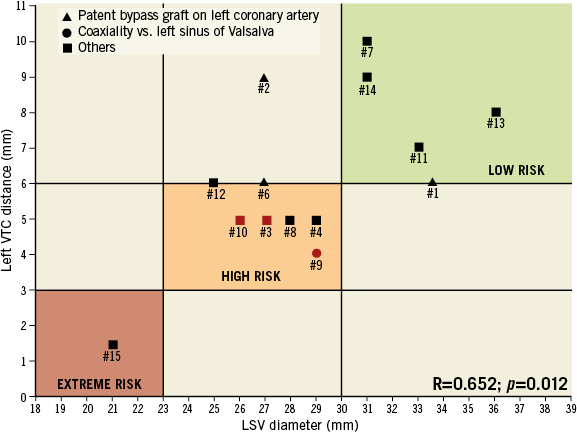
Figure 2. Patients undergoing baseline CT evaluation are reported according to left SV diameter and left VTC distance. Patients with patent bypass graft are shown with a triangle. Patients with coaxiality vs. left sinus of Valsalva are shown with a circle. Remaining patients are reported with a square. Patients who experienced coronary obstruction are displayed in red.
PROCEDURAL DATA
The transfemoral access was used in all but two subjects who received the transaortic approach. CV and EvR 23 mm were deployed in seven and eight patients, respectively. Three procedures were performed in an urgency scenario because of refractory pulmonary oedema.
A “preventive” LM stenting was successfully carried out using the “chimney technique” in patient #15. She had an LSV diameter similar to the external diameter of the bioprosthesis (21 mm) and a left VTC distance of 1.5 mm (Figure 1, Figure 2).
Post-dilation was performed in 10 cases (66.7%). The average implantation height was 8.2±3.7 mm without differences between old and new devices (p=0.777).
EARLY AND LATE CORONARY OBSTRUCTION
Acute and subacute LM obstruction occurred in two patients (#10 and #3), successfully treated with percutaneous coronary stenting using the “tunnel technique”. Both patients had a failed MF 21 mm with leaflet wear and tear as the mechanism of degeneration (involving the left leaflet in one case and non-coronary leaflet in the other one) and similar anatomic features (left VTC of 5 mm in both cases and LSV diameters of 26 and 27 mm, respectively).
In patient #10, the coronary obstruction occurred immediately after Evolut R 23 mm deployment with immediate cardiac arrest. During cardiopulmonary resuscitation, cannulas for CPB were placed in less than four minutes.
In patient #3, the LM occlusion occurred 50 minutes after the procedure with chest pain onset, anterolateral ST elevation followed by recurrent ventricular fibrillation. CPB was quickly started given the severe haemodynamic instability and the difficulty in LM cannulation.
At 20 days from the procedure, patient #11 was re-admitted due to refractory angina. Selective cannulation of the LM failed, but aortography showed a “contrast defect” in the LSV that was confirmed by the urgent CT. Therefore, an off-pump bypass left internal mammary artery (LIMA) graft on the left anterior descending (LAD) coronary was successfully performed.
Left VTC distance was significantly lower in patients who experienced LM occlusion as compared to those who did not (4.7±0.6 vs. 7.3±1.8 mm; p=0.002). Nevertheless, LSV diameter did not significantly differ between the two groups (27.7±1.2 vs. 30±3.4 mm; p=0.279).
RESIDUAL STENOSIS
Post-procedural mean gradient was 16.5±7 mmHg without differences between the on- and off-label groups (Figure 3). Four patients (26.7%) had mild residual stenosis (mean gradient between 20 and 40 mmHg) (Table 4), two in the on- and two in the off-label group; none had moderate or severe stenosis (>40 mmHg) (Figure 3). A trend of correlation between implantation height and post-procedural mean gradient was observed (R=0.492; p=0.062). At the 30-day follow-up, mean gradient remained relatively low (17.7±6.7 mmHg) with a mean effective orifice area (EOA) of 1.3±0.3 cm2 without differences when the population was stratified according to the procedure. Among patients who reached the one-year follow-up (66.7%), mean gradient and EOA were 16.5±8.8 mmHg and 1.3±0.4 cm2, respectively.

Figure 3. Post-procedural mean gradients, assessed by echocardiography before discharge, are shown for all patients according to the kind of procedure: on-label for Mitroflow 23 mm and off-label for Mitroflow 19 and 21 mm.

OTHER OUTCOMES
Complete VARC-2 composite endpoints are shown in Table 4.
A new permanent pacemaker was needed in two patients before discharge. One patient experienced a minor vascular complication (haematoma of the access site) without life-threatening bleeding.
Patients had none or mild prosthetic aortic regurgitation at all time points after the procedure. Functional NYHA Class II or I was observed in all cases.
A CT scan performed on patient #15 at the 30-day follow-up showed patency of the chimney stent.
No deaths, strokes or acute kidney injuries (AKIs) occurred during the follow-up.
Discussion
The main findings of the present study can be summarised as follows. First, TAVI with CV or EvR 23 mm in small MF bioprostheses has a favourable outcome up to the one-year follow-up. Second, a careful risk evaluation of coronary obstruction is mandatory and should be based on CT evaluation of parameters such as VTC distance and SV diameter. Third, the “chimney” technique is a feasible preventive treatment for patients at prohibitive surgical risk and extreme risk of coronary obstruction; it seems to be effective at short-term follow-up. Fourth, the standby for CPB is an essential tool to provide safe treatment of acute coronary obstruction (i.e., tunnel technique). Finally, the procedure is associated with low post-procedural gradients for both 23 mm and ≤21 mm MF sizes.
In VIVID, the largest registry on TAVI ViV procedures2,17, an increased mortality rate was observed among patients with small bioprostheses (about 25%) and those with failed MF (roughly 13%). In contrast to these results, we observed a favourable outcome without fatal events up to the one-year follow-up. This is probably due to the careful measures to prevent or deal with coronary events.
Coronary obstruction is the most feared complication for TAVI in degenerated surgical bioprostheses. The incidence of this adverse event is definitely higher (up to 3.5%) than that observed in TAVI on native valves (<1%)18. In our specific population (small Mitroflow), a roughly fivefold higher rate of LM occlusion was observed compared with the VIVID results.
Mitroflow is a stented bioprosthesis with leaflets mounted on the external side of the valve frame. This specific feature allows reducing bioprosthesis bulk and maximising the orifice area, improving the haemodynamic profile. Nevertheless, it results in a higher risk of coronary obstruction during TAVI because of bioprosthetic leaflet displacement towards the coronary ostia. Moreover, Mitroflow is usually implanted in a supra-annular position, promoting interaction between bovine leaflets and coronary ostia. As a consequence, a higher incidence of coronary complications should be expected in patients undergoing TAVI for failed MF as compared to other types of surgical bioprosthesis.
Without doubt, surgical redo must be considered as the first choice treatment for patients with MF degeneration. A percutaneous option should be considered for patients deemed not suitable for open surgery because of calculated high risk, frailty or poor general conditions.
However, the risk of coronary obstruction also depends on the aortic root anatomy. In this context, CT evaluation before TAVI is an essential tool for the risk assessment of this complication. In our selected population, the presence of low-lying coronary ostia did not impact on the risk of coronary obstruction because the coronary ostia were always below the MF posts.
Recently, the Vancouver group has reported the VTC distance as a new parameter for risk stratification of coronary obstruction during ViV12,13. VTC distance mainly depends on SV diameter and bioprosthesis coaxiality. In a patient with a capacious aortic root and a large LSV, left VTC distance should be high except in the case of a non-coaxial (tilted) bioprosthesis versus the left coronary sinus. On the other hand, in a subject with a non-capacious aortic root with a small LSV, the left VTC should be short. We observed a significant correlation between the left VTC distance and LSV diameter and a significantly lower left VTC in patients who experienced LM obstruction as compared to those who did not. According to these observations, we could speculate that: i) patients with a VTC distance <3 mm and an SV diameter very similar to the external MF diameter (≤23 mm) should be considered at extreme risk for coronary obstruction; ii) patients with a VTC >6 mm and an SV diameter >30 mm should be considered at low risk for coronary obstruction; iii) patients with VTC values between 3 and 6 mm and SV diameters between 24 and 30 mm should be deemed at high risk for coronary obstruction (Figure 2).
In the only patient at extreme risk of coronary obstruction, we successfully performed the “chimney technique” that resulted in being effective at the one-month follow-up. To the best of our knowledge, this is the first report on preventive LM stenting as a treatment strategy during a ViV procedure. It should be taken into account that this technique does not make LM re-cannulation practicable. Moreover, follow-up is limited and our patient was treated with triple antithrombotic therapy (a new oral anticoagulant and dual antiplatelet therapy) that may promote stent patency at the 30-day follow-up. Therefore, the “chimney technique” should be carried out in selected patients deemed at prohibitive risk for surgical redo and deserving TAVI ViV although at extreme risk for coronary occlusion.
Pre-emptive positioning of a coronary stent in the left coronary for LM protection has been previously reported19,20. However, we observed a subacute case of LM occlusion (50 minutes after the procedure) that would not have been treated using this approach. Moreover, the presence of the guiding catheter and wire in the coronary artery could hide the evidence of acute obstruction at the aortography due to bioprosthesis leaflet dislocation.
When acute or subacute coronary occlusion occurs, the coronary ostium cannulation may not be easy and several manipulations could be necessary to obtain a successful result. In this context, the presence of a CPB “ready for use” could make the difference for patient survival.
The displacement of the bioprosthesis leaflets towards the coronary ostia was the mechanism of both acute and subacute LM occlusion. In both cases, wear and tear was observed, which probably simulated a “flag effect” on the LM ostium.
Instead, late coronary obstruction, observed 20 days after the ViV procedure, was associated with different mechanisms. Non-coaxiality towards the left sinus was observed at the baseline CT. Therefore, a “slow-flow chamber” in front of the LM ostium was created by CoreValve deployment with probable consequent left sinus thrombosis (contrast defect at CT) and LM flow reduction. Given that unstable angina was refractory to medical therapy and LM cannulation was unsuccessful, coronary revascularisation with off-pump CABG was necessary. However, after anticoagulation therapy, a reduction of the contrast defect at the CT was noted, confirming the thrombotic nature of the image and suggesting that anticoagulation therapy might be used in selected patients after TAVI ViV.
Diemert et al reported low residual mean gradients in 16 patients with small surgical bioprostheses (two MF) treated with Evolut R 23 mm21. Our results confirm these previous observations and are also in line with those reported in the VIVID registry2. Interestingly, we observed similar mean gradients in patients with MF 23 mm (on-label procedure) compared with those undergoing off-label intervention (MF 19 and 21 mm).
Moreover, we found a trend of correlation between implantation height and post-procedural mean gradient. This observation is in agreement with recent in vitro and clinical results15,22, confirming that a high implantation would improve the haemodynamic profile after TAVI ViV.
Limitations
This study presents several limitations. First, there is the small size of the study population and the low number of events (i.e., coronary occlusion) which limited the statistical analysis. Second, there is the retrospective nature of the study and the lack of a concurrent control arm. Third, this was a single-centre experience. Finally, the one-year follow-up was reached by only two thirds of the patients. For all these reasons, our results should be interpreted cautiously. Further investigations on larger and multicentre populations are necessary in order to endorse our observations.
Conclusions
TAVI with CV or EvR 23 mm in failed small MF is safe and effective provided that there is a careful work-up including risk evaluation and adoption of preventive measures for coronary occlusion. Low mean gradients can also be obtained in off-label procedures.
| Impact on daily practice CoreValve or Evolut R implantation is a feasible, safe and effective treatment option for patients with degenerated small Mitroflow bioprostheses who are deemed not suitable for surgical redo because of comorbidity or frailty. A careful evaluation of risk for coronary occlusion using computed tomography is mandatory. Standby for cardiopulmonary bypass during the procedure could be an essential tool to treat coronary complications safely. Preventive left main stenting might be reserved to patients at extreme risk for coronary obstruction. Low gradients can be obtained regardless of Mitroflow size. |
Conflict of interest statement
F. Ettori is a proctor physician for Medtronic. M. Rinaldi is Global Senior Technical Lead for Medtronic Heart Valve Therapies. The other authors have no conflicts of interest to declare.

