Abstract
The earliest evidence supporting transcatheter aortic valve implantation (TAVI) was derived from its comparison with conservative therapy in inoperable patients and with surgical aortic valve replacement (SAVR) in extremely high risk patients. TAVI had a relative advantage in these situations being less invasive and hence less prone to the classic early postoperative devastating complications. To prove as effective in less fragile and less morbid patients, the long-term durability of the haemodynamic and clinical gains from TAVI needs to be confirmed.
In this report we will discuss three aspects of the dilemma of expanding TAVI indications to lower-risk patients: first, available data on early and late outcomes after TAVI; second, durability issues; and third, TAVI complications and procedural refinements.
Early outcomes
When looking at the three large randomised clinical trials that have compared TAVI vs. SAVR, the following phenomenon (Figure 1) is noticeable. As the STS-predicted risk of mortality (PROM) score went down, the 30-day mortality gap between SAVR and TAVI became progressively narrower, from PARTNER IA trial1 (STS-PROM: 11.7 and 11.8 for SAVR and TAVI arms, respectively; TAVI gave an absolute risk reduction of 2.8%) to CoreValve US pivotal trial2 (STS-PROM: 7.5 and 7.3; TAVI gave an absolute risk reduction of 1.2%) to PARTNER IIA trial3 (STS-PROM: 5.8 for both SAVR and TAVI arms; TAVI gave an absolute risk reduction of only 0.2%). It turns out that, as the overall predicted risk (using the surgical risk scores) decreases, 30-day post-TAVI mortality gets closer to the PROM, and TAVI cannot outperform SAVR anymore. In this case, long-term durability of benefit from TAVI represents the main determinant of its efficacy.
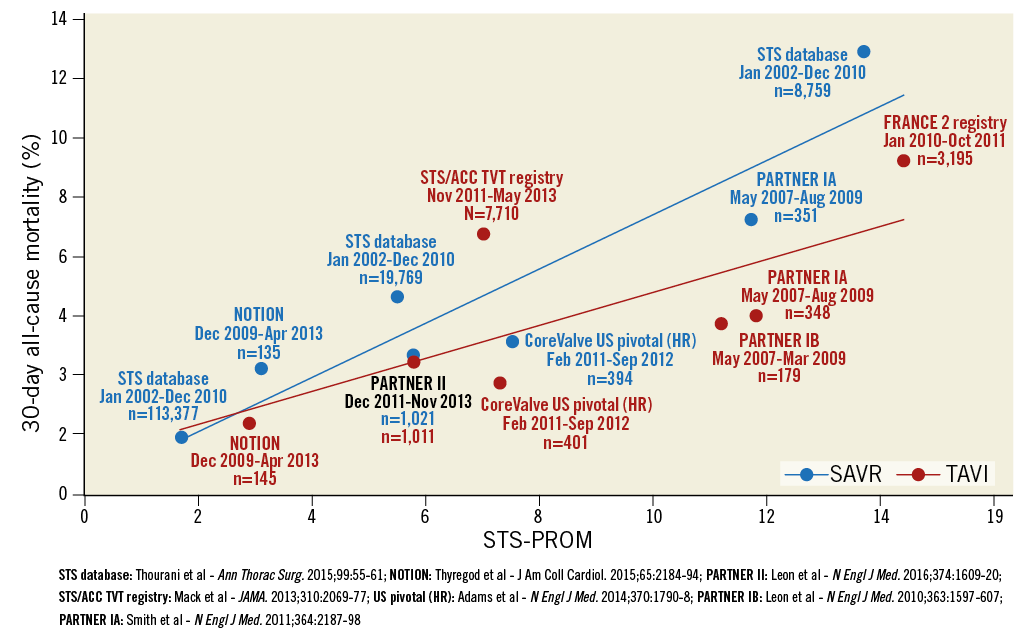
Figure 1. Thirty-day all-cause mortality after TAVI and SAVR plotted against a decreasing risk of tested patients. Data include i) RCTs, namely PARTNER IA, PARTNER IB, PARTNER II, CoreValve US pivotal (HR) and NOTION trials, and ii) sponsor-independent all-comer registries with mandatory data collection on a national scale, namely FRANCE 2, TVT and GARY registries for TAVI and STS database for SAVR. Modified with permission from Abdelghani et al, Circ Cardiovasc Interv. 2016;9:e002944.
Late outcomes and durability issues
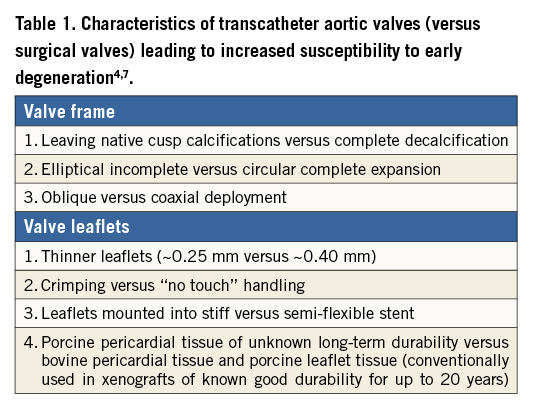
In order to offer the patient a lifelong cure by performing a single intervention, the durability of a heart valve should exceed the life expectancy of the patient. Factors associated with decreased xenograft durability include not only patient-related factors (e.g., younger age, male gender, renal impairment, recurrent infection, and immunosuppression) but also valve/procedure-related factors (e.g., crimping, non-circularity and non-coaxiality of deployment as well as paravalvular/transvalvular aortic regurgitation)4 (Table 1). Up to now, few reports on structural valve deterioration (SVD) of transcatheter aortic valves (TAVs) exist. Given the fact that SVD usually occurs beyond 6-10 years after surgery (median time from SAVR to reintervention for SVD is 9 [IQR, 6-12] years5) and that reliable long-term data after TAVI are currently available only up to five years, there is currently no guarantee that the implanted TAVs are durable enough. An illustrative example is what happened with the Mitroflow pericardial surgical bioprosthetic valve (previously Sorin Group, now LivaNova, London, United Kingdom), where freedom from SVD was 97% after five years, but only 39% at 10 years6.
TAVs differ from surgical prosthetic valves in many respects, summarised in Table 1, and collectively make durability concerns more relevant in the setting of TAVI. In an experimental fatigue simulation test subjecting transcatheter and surgical bioprosthetic valves to identical loading conditions, TAV leaflets sustained higher stress, strain, and fatigue damage7. The results suggested that durability of TAVs could be significantly reduced – to about 7.8 years compared to 16 years for surgical valves subjected to the same conditions.
TAVs vary in their leaflet precursor tissue and could be classified into balloon-expandable bovine pericardial tissue (SAPIEN XT and SAPIEN 3; Edwards Lifesciences, Irvine, CA, USA), self-expanding porcine pericardial tissue (CoreValve®, Evolut R® [both Medtronic, Minneapolis, MN, USA], and ACURATE neo™ [Symetis, Ecublens, Switzerland]), self-expanding bovine pericardial tissue (Portico™; St. Jude Medical, St. Paul, MN, USA), self-expanding native porcine leaflets (ACURATE TA™; Symetis), and alternative expansion design bovine pericardial tissue (Direct Flow® [Direct Flow Medical, Santa Rosa, CA, USA] and Lotus™ [Boston Scientific, Marlborough, MA, USA])4. SVD mechanisms vary, being, for example, basically due to calcification leading to stiffening and stenosis in bovine pericardial valves and to tear leading to insufficiency in porcine valves4.
Permanent pacing and related cardiopathy
A trade-off has been observed – possibly incidentally – between the rates of paravalvular aortic regurgitation (AR) and conduction defects after implementation of the newer TAVI devices. In the PARTNER IA trial1, the use of the first-generation balloon-expandable device was associated with a 30-day risk of permanent pacemaker implantation (PPI) of 3.8% and of moderate-severe AR of 12.2%. In the PARTNER IIA trial (involving the second-generation balloon-expandable device), 8.5% of patients required PPI and 3.7% had moderate-severe AR at 30 days3. The third-generation balloon-expandable device (SAPIEN 3) has been consistently shown to be associated with more efficient paravalvular sealing but a higher risk of PPI than the second-generation device8. The mechanically expandable repositionable Lotus valve with a very efficient paravalvular sealing has also been shown to be associated with a markedly higher risk (>30%) of PPI than other technologies9.
Another increasingly recognised phenomenon is the high rate of spontaneous resolution of conduction defects among post-TAVI pacemaker recipients. In these patients, pacemaker dependency after TAVI has been reported at a rate of only 37%10 in the short term and 39% in the long term11. The exact magnitude of this phenomenon is variable among reports, reflecting the heterogeneity of the indications for PPI between centres and operators.
Data on pacing-induced cardiopathy are conflicting, with some studies reporting a life expectancy among permanent pacemaker recipients that is comparable to that of the general population12. Other studies have shown, however, that prolonged pacing leads to ventricular dyssynchrony and adverse left ventricular remodelling13, eventually leading to adverse cardiovascular outcomes13. In TAVI patients, the adverse influence of pacing is difficult to appreciate. On one hand, patients with a new or prior permanent pacemaker in the PARTNER I trial were shown to have significantly longer hospitalisation14, higher mortality and re-hospitalisation and lower ejection fraction at one year15. On the other hand, many other studies of the impact of PPI on post-TAVI outcomes generally involved short follow-up time and low percentage of cumulative pacing (due to the low rate of pacemaker dependency) and, not surprisingly, the majority of these studies failed to demonstrate a negative effect of PPI on clinical outcomes16,17. While it was infrequent to show a negative impact of a new PPI on one to two-year outcomes, a new left bundle branch block (LBBB) has consistently been shown to portend a worse prognosis18.
At least three conclusions can be derived from the aforementioned data. 1) The current technologies and the accumulated experience are not sufficient to reduce the risk of new conduction defects after TAVI, emphasising the need for more investment from scientists and industry to tackle this shortcoming of TAVI. 2) The criteria for – and the timing of – PPI after TAVI should be adjusted and standardised taking into consideration the high rate of conduction defect resolution and pacemaker independency among pacemaker recipients after TAVI. 3) Including new persistent conduction abnormalities (most importantly, LBBB) as a safety endpoint after TAVI rather than only PPI would improve the consistency of the data on new conduction defects complicating TAVI.
TAVI the procedure, made effective, safe and simplified
EFFICACY AND SAFETY
The PARTNER IIA trial (n=1,011 in the TAVI arm, transfemoral [TF] in 76%, enrolled in the period from December 2011 to November 2013) showed that TAVI performed with the use of a SAPIEN XT valve was non-inferior to SAVR with respect to death from any cause or disabling stroke at two years3. Patients treated with TAVI had fewer bleeding events, less acute kidney injury (AKI) and new-onset atrial fibrillation (AF), as well as a more rapid recovery and a shorter ICU and hospital stay than patients treated with SAVR3.
In the manufacturer-sponsored multicentre SAPIEN 3 registry (TF in 88%)19, 1,078 intermediate-risk patients were enrolled between February and December 2014. In this more contemporary series using the third-generation balloon-expandable valve technology, although baseline patient characteristics were similar to those of patients included in the PARTNER IIA trial, 30-day and one-year outcomes were noticeably improved. All-cause mortality was as low as 1.1% at 30 days and 7.4% at one year (vs. 3.9% and 12.3% in PARTNER IIA). Re-hospitalisations were also fewer, being 4.6% at 30 days and 11.4% at one year (vs. 6.5% and 14.8%). At 30 days, the rates of stroke (2.7% vs. 3.2%), myocardial infarction (MI, 0.3% vs. 1.2%), major vascular complications (6.1% vs. 7.9%), life-threatening and disabling bleeding (4.6% vs. 10.4%) and new AF (5% vs. 9.1%) were all numerically lower. In a propensity score analysis of intermediate-risk patients20, treatment with SAPIEN 3 (in patients from the SAPIEN 3 registry19) was shown to be significantly superior to SAVR (in patients from the PARTNER IIA trial3) for the composite of death from any cause, all strokes, and incidence of moderate-severe AR.
In the largest European experience, the annual German national rate of severe complications (defined as death on the day of intervention, conversion to sternotomy, low cardiac output that required mechanical support, aortic dissection, or annular rupture) showed a significant decrease over time from 6.8% (in 2011) to 4.9% (in 2012) and 3.9% (in 2013) (p<0.001)21. However, the regression in the proportion of patients with in-hospital death showed a less remarkable decline from 5.9% (in 2011), to 5% (in 2012), and 4.9% (in 2013) (p=0.078). In a systematic review22 of studies using second-generation valves published up to January 2015 and after exclusion of first-in-man studies, all-cause 30-day mortality occurred in 4.7%, MI in 1.5%, AKI stage 3 in 2.8%, life-threatening bleeding in 4.0%, major vascular complications in 4.3%, major stroke in 2.1%, PPI in 13.4% and moderate-severe AR in 2.9% of patients.
MINIMALIST TAVI
The minimalist approach of TAVI implies a tailored approach to each patient based on individual risk stratification, shifting to a default practice of transfemoral access, local anaesthesia and early mobilisation. This approach also implies restrictive and selective use of hybrid operating rooms, surgical cut-downs, urinary catheters, central venous catheters, radial artery lines and other invasive monitoring equipment, general anaesthesia, transoesophageal echocardiography, and temporary pacing after the procedure in the absence of new significant conduction defects. In this approach, choosing the degree of invasiveness and the complexity of periprocedural interventions is based on patient characteristics rather than the institutional practice and operator’s preference.
Feasibility of minimalist TAVI has been shown at equivalent safety and efficacy to the standard approach and to be associated with shorter length of hospitalisation and lower resource use23.
In the European experience, the ESC Transcatheter Valve Treatment Sentinel Registry reported in 2014 a rapid shift of procedural sedation from general to local anaesthesia, with the latter being applied to 37.5% of procedures in 2011 and to 57% in 2012 while maintaining procedural safety24. Local anaesthesia with sedation as compared to general anaesthesia has been shown to guarantee equivalent safety and efficacy of TAVI at a shorter procedure time, lower labour costs and earlier mobilisation of patients25. Transthoracic echocardiography-guided TAVI under sedative anaesthesia has also been shown to be feasible and safe compared with transoesophageal echocardiography-guided procedure26. Procedural time was shorter with no difference in procedural success, severity of paravalvular AR, need for additional valve implantation or periprocedural complications (including stroke and death). Evidence also supports a selective omission of ICU admission27.
It is now clear that TAVI can be simplified in the majority of patients. The challenge is how to stratify patients in an efficient way that safely allocates patients to the minimalist “default” approach or to a more “conservative” (previously “standard”) approach. Mortality after TAVI cannot be accurately predicted using the PROM scores derived from, and efficiently performing in, surgically treated patients. Major periprocedural complications (carrying a risk of up to 60% mortality21), which albeit are encountered progressively less, can still affect around 4% of TAVI patients21. These devastating complications are sometimes difficult to predict based on patients’ baseline characteristics28.
The ongoing “Multidisciplinary, Multimodality, But Minimalist Approach to Transfemoral Transcatheter Aortic Valve Replacement (3M TAVR)” single group assignment trial (NCT02287662) targets the demonstration of a clearer gain and more confident safety of minimalist TAVI. The composite of all-cause mortality, major stroke or life-threatening bleed at 30 days and one year are the primary outcome measures. The study started in January 2015 and targets enrolling 1,200 patients.
Conclusion
In order to maintain the confidence in TAVI as a safe, effective and ever-improving therapy, the following points are to be considered. 1) The planning process should be kept comprehensive and multidisciplinary. 2) Simplification of the procedure and standardising the minimalist practice into a “default” strategy should continue side by side with optimising identification of patients who could benefit from a more conservative approach. 3) Extension of the indications of TAVI should not involve younger patients until the durability question has been unequivocally answered.
These recommendations could be useful until solid evidence becomes available supporting TAVI implementation in low-risk patients. For the latter, a co-primary endpoint that combines medical non-inferiority and cost-effectiveness superiority could serve as a practical outcome measure.
Conflict of interest statement
P.W. Serruys is the chairman of the SURTAVI trial. M. Abdelghani has no conflicts of interest to declare.

