
Abstract
Aims: The Evolut PRO is a new transcatheter heart valve with an outer pericardial wrap intended to reduce paravalvular leak and facilitate tissue ingrowth. We aimed to evaluate the clinical performance and safety of the Evolut PRO valve in standard practice.
Methods and results: FORWARD PRO is a prospective, multinational, multicentre observational study. Transcatheter aortic valve implantation with the Evolut PRO valve (23, 26, or 29 mm) was attempted in 629 non-consecutive patients from 39 centres from February 2018 to January 2019. The primary endpoint was the rate of all-cause mortality at 30 days compared to a pre-specified performance goal. An independent clinical events committee adjudicated safety endpoints based on VARC-2 definitions. All echocardiograms were centrally assessed by an independent core laboratory (Mayo Clinic, Rochester, MN, USA). Baseline characteristics included mean age 81.7±6.1 years, 61.8% female, STS score 4.7±3.3%, and 33.6% were frail. All-cause mortality at 30 days was 3.2%, which was lower than the pre-specified performance goal of 5.5% (p=0.004). Greater than mild AR was present in 1.8% of patients at discharge.
Conclusions: The FORWARD PRO study confirmed the safety and efficacy of the Evolut PRO transcatheter aortic valve system with an external pericardial wrap. ClinicalTrials.gov Identifier: NCT03417011
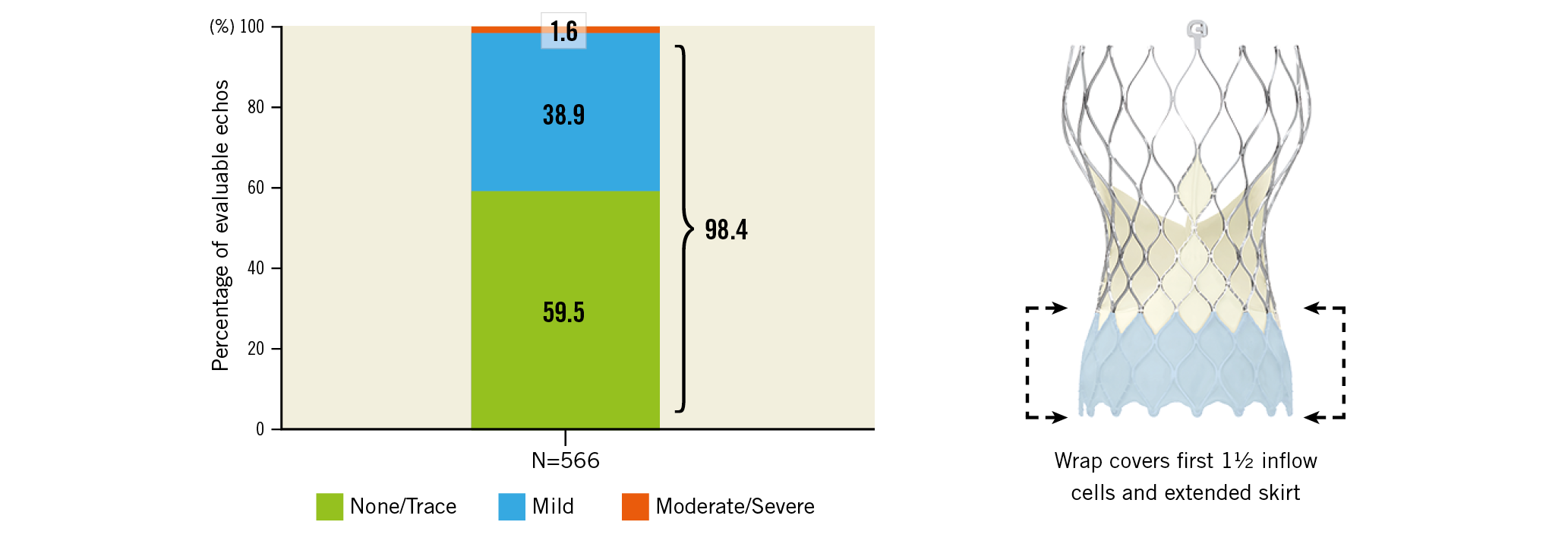
Visual summary. Paravalvular leak at discharge and (right) the Evolut PRO valve (Medtronic) with external pericardial wrap. Reproduced with permission from Medtronic, Inc.
Introduction
Procedure planning with computed tomography (CT), optimal valve size selection, repositionable valve technology and adherence to “best practices” to optimise transcatheter aortic valve implantation (TAVI) have improved overall outcomes and the rates of paravalvular leak (PVL), compared with early TAVI studies1,2. However, while in the intermediate- and low-risk studies3,4,5,6 the overall mortality outcomes were similar between TAVI and surgery, the rates of PVL were lower with surgical valves.
Technological iterations are being made to mitigate further the risk of PVL. The addition of an external pericardial wrap to the Evolut™ R valve resulted in the next-generation Evolut™ PRO transcatheter aortic valve system (both Medtronic, Minneapolis, MN, USA). The Evolut PRO system retains all the characteristics of its predecessor (supra-annular valve function, repositionable and low-profile delivery system via the InLine sheath [Medtronic]) and has an outer pericardial wrap at the inflow portion of the valve.
Initial use of the Evolut PRO valve in 60 patients in the USA showed that 72.4% of patients had no or trace aortic regurgitation (AR) at 30 days7, whilst maintaining the safety profile of the procedure. Use of the Evolut PRO valve in a large cohort of patients from the United States Society of Thoracic Surgeons/American College of Cardiology/Transcatheter Valve Therapy Registry (US STS/ACC/TVT Registry) also showed that, compared to the Evolut R valve, the Evolut PRO valve had lower rates of AR at 30 days8.
The FORWARD PRO study evaluated the clinical performance and safety of the Evolut PRO valve in a large cohort of patients using standard clinical practice.
Methods
STUDY DETAILS
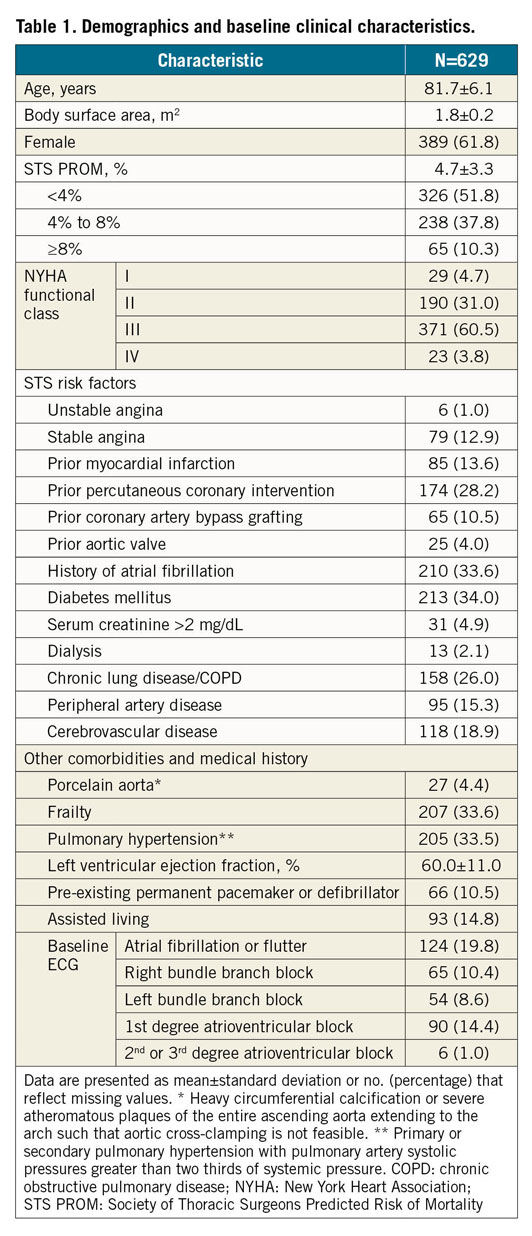
FORWARD PRO is a prospective, single-arm, multicentre, interventional post-commercialisation study. Patient selection was based on the Evolut PRO system instructions for use. Sites were instructed to enrol consecutive patients and use a Medtronic valve. Eligible patients had symptomatic native aortic valve (AV) stenosis or a failed surgical AV bioprosthesis (stenotic and/or insufficient) requiring replacement, and were at high or greater risk for surgery per the Heart Team, or were >75 years old and at intermediate risk for surgery (STS PROM ≥4% or with an estimated hospital mortality ≥4% per the Heart Team). Additional conditions such as frailty were considered9. Emergency procedures and patients with a life expectancy of <1 year were not allowed.
An independent clinical events committee (CEC) adjudicated deaths and safety endpoint-related events. The study sponsor (Medtronic) performed risk-based monitoring that included 100% monitoring of patient consent, primary and secondary related endpoints and study-specific adverse events. Patients underwent clinical evaluations at baseline, hospital discharge and 30 days and will be followed for five years.
The FORWARD PRO steering committee (G. Manoharan, E. Grube, N. Van Mieghem, P. Lancellotti, L. Søndergaard, C. Tamburino, S. Windecker) designed the study in collaboration with the sponsor and had oversight of study activities. The study followed the principles of the Declaration of Helsinki and all patients signed an informed consent form. The corresponding author decided to submit this manuscript for publication.
STUDY DEVICE
The Evolut PRO is a repositionable, self-expanding supra-annular valve within a nitinol frame. It was available in three sizes during the study (23, 26 or 29 mm) to treat aortic annuli diameters of 18–26 mm. An outer porcine pericardial wrap, 1.5 cells in height, was sutured to the inflow portion of the valve frame to diminish post-TAVI PVL. To assist with accurate positioning, the valve can be partially or fully recaptured until released from the delivery system. The valve could be implanted using a 16 Fr equivalent EnVeo™ R InLine Sheath (Medtronic) in access vessels with a diameter ≥5.5 mm or a 20 Fr standard sheath in diameters ≥6 mm. The decision to use the InLine or a separate introducer sheath was at the operators’ discretion. Implant procedures were performed per the standard procedures of the implanting physicians.
STUDY PROCEDURES
All patients were required to undergo multislice CT prior to the procedure for proper device sizing and procedural planning. Transthoracic echocardiography (TTE) was performed prior to the procedure and before hospital discharge and centrally assessed by an independent core laboratory (Mayo Clinic, Rochester, MN, USA). An integrated approach using colour flow, pulsed-wave and continuous-wave Doppler was used to assess the severity of AR10. The unifying 5-class grading scheme was used to grade AR11. The modified Rankin Scale12 was scored at baseline, discharge, every follow-up visit and 90 days after the onset of any confirmed or suspected neurological event.
ENDPOINTS
The primary endpoint was the rate of all-cause mortality at 30 days compared with a pre-specified performance goal of 5.5%, the upper two-sided 95% limit from the Evolut R US Study13. Secondary endpoints included the proportion of patients with
STATISTICAL ANALYSIS
The primary analysis cohort for this study comprises patients who underwent attempted implant of an Evolut PRO valve. Haemodynamic assessments are reported for implanted patients. For the primary endpoint, a sample size of 564 patients was required to attain 90% power in a one-sided test at the 0.05 level of significance, which was increased to 600 to account for attrition. The one-sided exact binomial test was performed for the primary endpoint. Additionally, the Kaplan-Meier event rate for 30-day all-cause mortality is presented.
The secondary haemodynamic endpoint of the incidence of no/trace AR at discharge was compared to a one-sided test at the 0.025 significance level. Baseline categorical variables are presented as counts and frequencies, and continuous variables as mean±standard deviations. Event rates are reported as Kaplan-Meier estimates. Statistical analyses were performed using SAS software, version 9.4 (SAS Institute, Cary, NC, USA).
Results
PATIENTS
There were 638 patients enrolled at 39 centres in 14 countries between February 2018 and January 2019, of whom 629 underwent an attempted implant (Figure 1). The mean age was 81.7±6.1 years, 61.8% were female, and the mean STS PROM was 4.7±3.3%. Most patients (64.3%) were in NYHA functional Class III or IV and 33.6% were frail (Table 1).
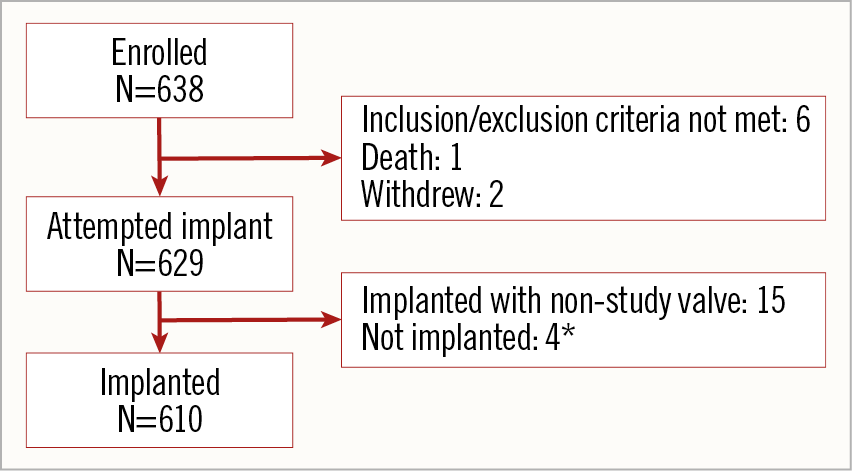
Figure 1. Patients. The primary analysis cohort for outcomes was the attempted implant cohort and for echocardiographic outcomes it was the implanted cohort. *See text for details.
PROCEDURAL CHARACTERISTICS
Of the 629 patients, 610 (97.0%) were implanted with the Evolut PRO valve, 15 patients received a non-study valve, and 4 were not implanted with a TAV; 1 had a major vascular complication requiring surgical repair, 1 experienced a femoral dissection, 1 had a cardiac tamponade after predilation during the procedure, and 1 patient only underwent percutaneous coronary intervention (Figure 1). These 19 patients were followed for 30 days and then exited from the study.
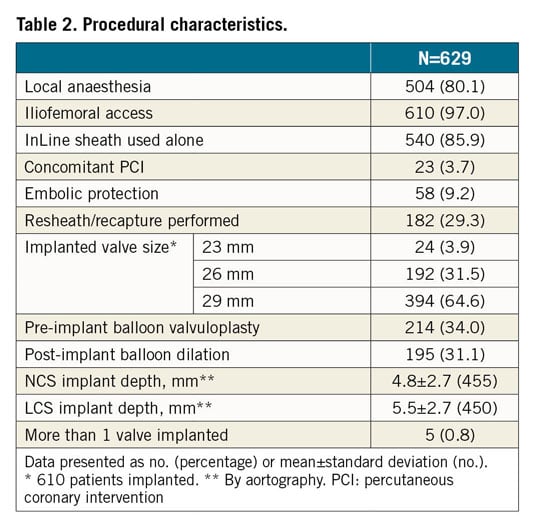
Most patients were implanted via iliofemoral access (97.0%) using local anaesthesia/conscious sedation (80.1%) (Table 2). The repositioning function of the Evolut PRO valve was used in 182 patients (29.3%). Pre-implant balloon valvuloplasty was performed in 34.0% and post-implant dilation was performed in 31.1% of patients. There were 5 patients (0.8%) who received 2 valves during the procedure; these were due to valve embolisation in 2 patients, valve migration in 1, and ectopic valve deployment in 2 patients.
30-DAY ENDPOINTS AND OUTCOMES
The primary endpoint of all-cause mortality at 30 days was 3.2% (one-sided 95% upper confidence interval [CI] = 4.6%), significantly lower than the performance goal of 5.5%, p=0.004 (Table 3, Figure 2). Cardiovascular death occurred in 2.7% of patients. The VARC-2 composite safety endpoint rate was 9.4% (Table 3).
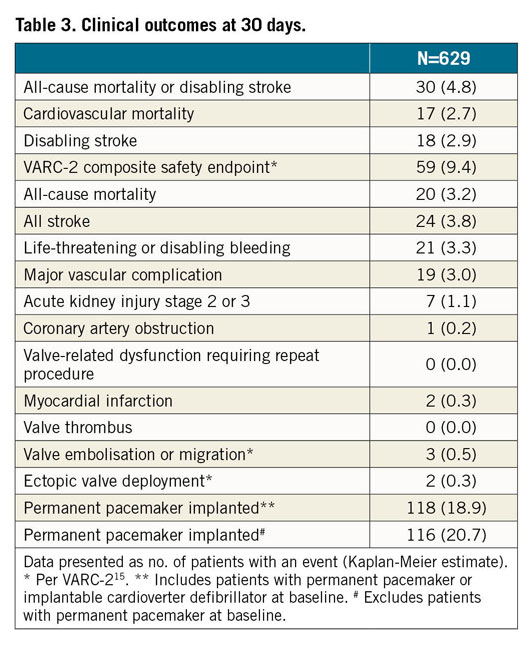
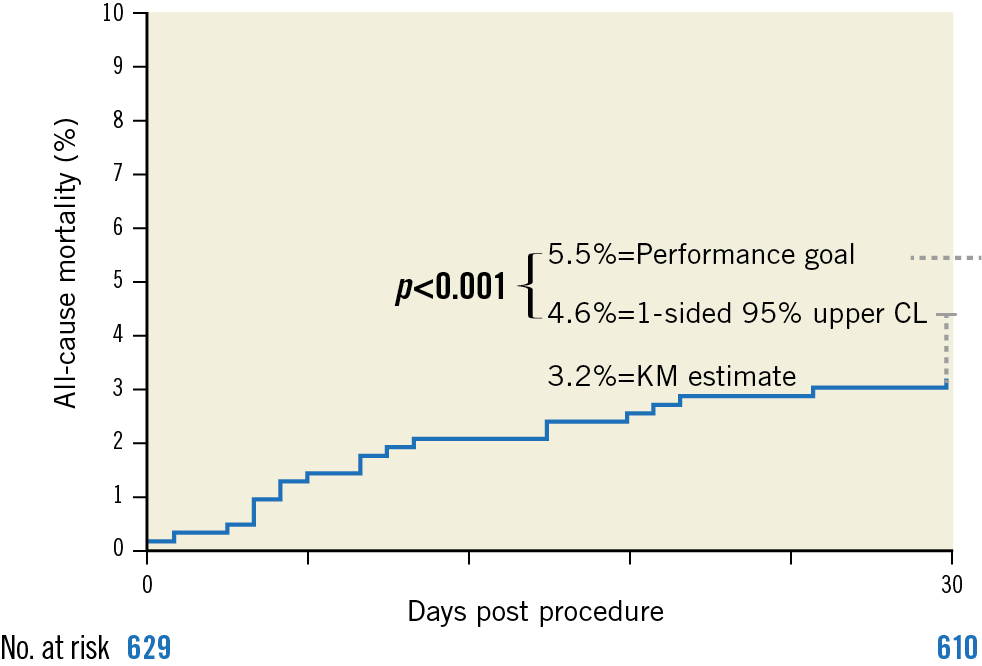
Figure 2. Primary endpoint of all-cause mortality at 30 days. Kaplan-Meier estimate of all-cause mortality. CL: confidence limit
Disabling stroke was observed in 18 patients (2.9%), 7 of whom died within 30 days. The major vascular complication rate was 3.0%. During the procedure, 1 patient experienced a coronary occlusion (0.2%). There were no cases of valve-related dysfunction requiring a repeat procedure. A new permanent pacemaker was implanted in 116 patients (20.7%), of whom 25 (21.7%) had right bundle branch block at baseline. The NYHA class improved in most patients (77.9%), with 55.4% of patients reported in NYHA Class I at 30 days (Figure 3).
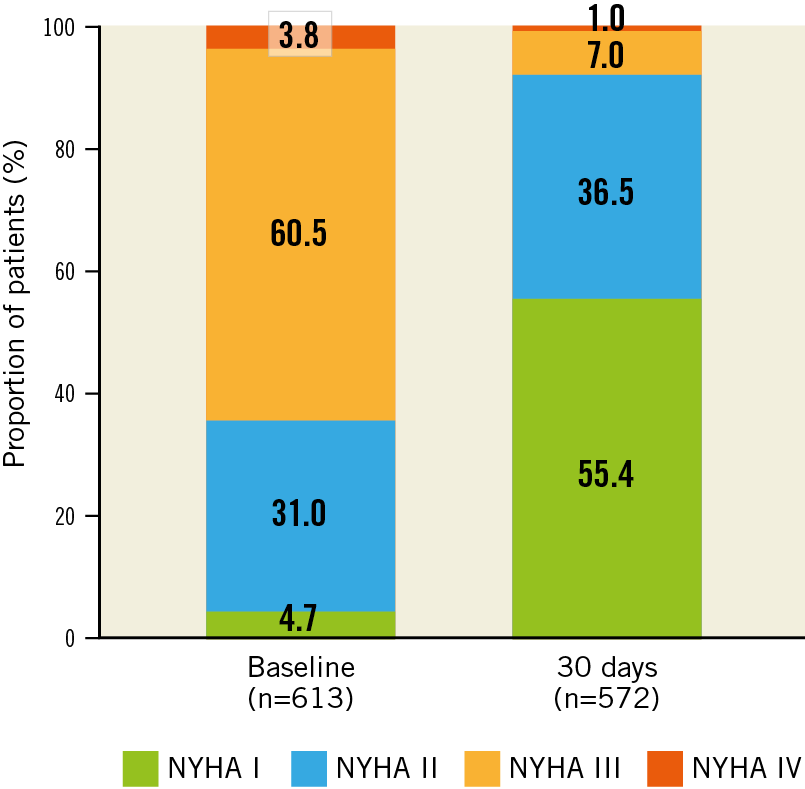
Figure 3. New York Heart Association functional class to 30 days.
HAEMODYNAMICS
At discharge, the mean aortic valve gradient (AVG) was 7.9±4.7 mmHg and the mean effective orifice area (EOA) was 2.1±0.6 cm2 (Figure 4A). The secondary endpoint of no or trace AR was 59.2% (one-sided 97.5% lower confidence limit of 55.1%), as compared to the performance goal of 67.1% (p>0.99), and was not met. The rate of moderate or severe AR was 1.8% (Figure 4B). Severe PPM was present in 17 patients (4.4%), with no differences in the distribution of the severity of PPM between men and women.
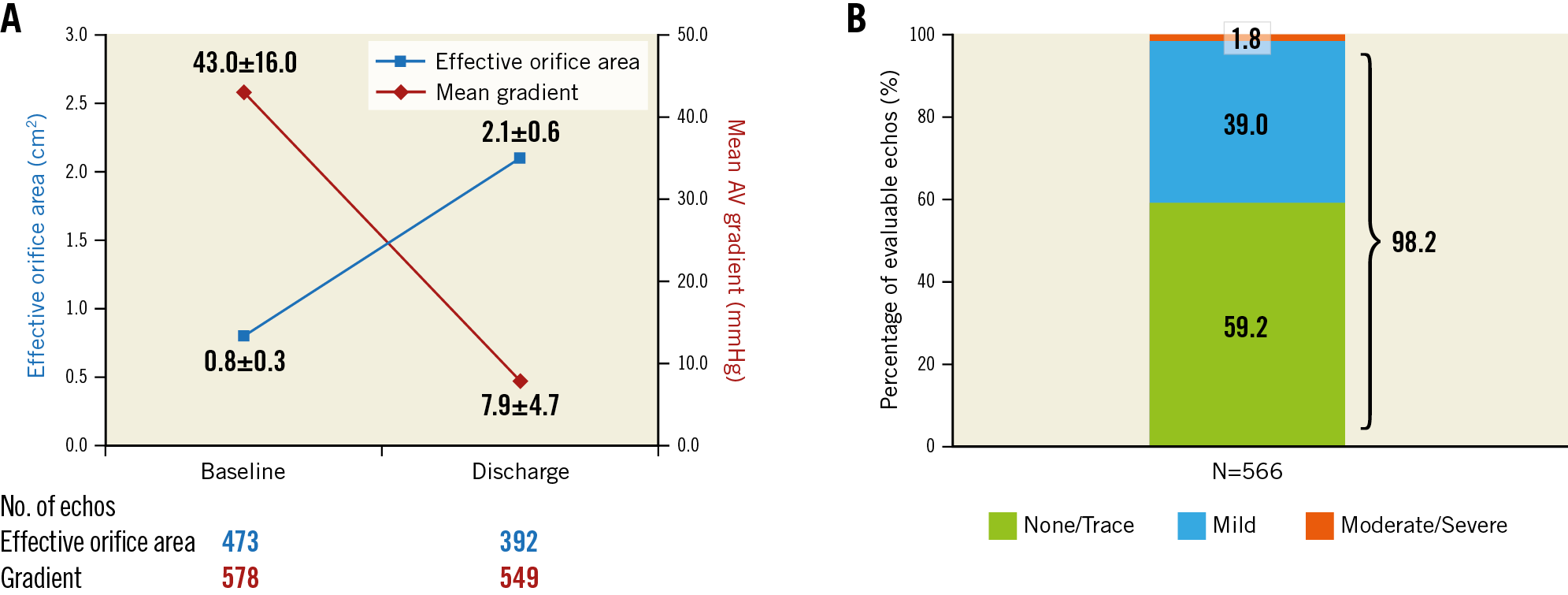
Figure 4. Aortic valve haemodynamics as assessed by independent core laboratory. A) Aortic valve EOA and AVG at baseline and discharge. B) Severity of AR. Values reported as mean±standard deviation. 98.2% of patients had less than mild AR.
Discussion
We report 30-day procedural and clinical outcomes from the FORWARD PRO study, the largest study of the Evolut PRO valve with independent echocardiographic core laboratory assessments and independent CEC-adjudicated results. Key findings include lower than expected all-cause mortality of 3.2%, a low disabling stroke rate of 2.9%, a low major vascular complication rate of 3.0% and favourable aortic valve haemodynamics, with an AVG of 7.9±4.7 mmHg and EOA of 2.1±0.6 cm2, and moderate or severe AR of 1.8%. However, the predefined performance goal of no or trace AR was not met.
The 30-day mortality rate of 3.2% in our study was significantly lower than the pre-specified performance goal of 5.5%, and lower than the STS PROM of 4.7±3.3%. This was comparable to reports of self-expanding and balloon-expandable valves in intermediate-risk patients3,6,14.
The addition of an outer pericardial wrap to mitigate PVL increased the device outer diameter by two Fr sizes (currently 16 Fr equivalent). Despite this, the rate of major vascular complications remained low at 3.0%. This was lower than previous reports from real-world use of the Evolut R in the FORWARD study (6.5%)14 or the SAPIEN 3 valve (Edwards Lifesciences, Irvine, CA, USA) in the SOURCE 3 Registry (4.1%)16. This may be due in part to the smaller outer diameter and resultant trauma caused by advancing the delivery system “sheathless”, using the InLine delivery system in most patients (85.9%) and increased operator experience in managing vascular access. The rate of life-threatening or disabling bleeding was also low (3.3%).
It has been hypothesised that the external pericardial wrap could lessen desired friction when interacting with the surrounding tissue, resulting in reduced stability of the valve during deployment. However, the proportion of patients in whom the resheath/recapture feature was used in the FORWARD PRO study was 29.3%, comparable to that reported from the FORWARD study of the Evolut R valve (25.6%)14. Additionally, the incidence of valve embolisation or migration was similar in this study of the Evolut PRO valve (0.5%) and in the FORWARD study of the Evolut R valve (0.9%). There were no patients who required a repeat procedure. All these observations would suggest that the stability of the Evolut PRO system is not affected by the pericardial wrap.
A disabling stroke was observed in 18 patients (2.9%) in the present study which is a similar rate to other contemporary TAVI studies of the same valve8,14. The rate was 1.4%, 3.2% and 1.2% in the FORWARD study, Placement of Aortic Transcatheter Valves (PARTNER) 2 trial and the Surgical Replacement and Transcatheter Aortic Valve Implantation (SURTAVI) trial, respectively3,6,14. There were no strokes in patients treated using an embolic protection device; however, the numbers are too small to draw meaningful conclusions. Coronary artery occlusion was reported in one patient. There was no incidence of annular rupture or valve thrombosis observed in the FORWARD PRO study.
HAEMODYNAMIC ASSESSMENTS
All AV echocardiographic measures in the FORWARD PRO study were core laboratory assessed. Previous generations of the self-expanding supra-annular valve have produced excellent haemodynamics post deployment, with discharge AVG and EOA in the CoreValve US Pivotal Extreme Risk Trial of 9.6±4.4 mmHg and 1.9±0.6 cm2, respectively17. Similar findings were observed in the FORWARD study using the Evolut R valve (8.5±5.6 mmHg and 1.9±0.6 cm2, respectively)14. It has been hypothesised that the external wrap on the Evolut PRO device could adversely impact the forward flow haemodynamics. Reassuringly, we observed low AVGs (7.9±4.7 mmHg) and EOAs (2.1±0.6 cm2) in our study, consistent with other reports of the Evolut PRO valve7,8. Moreover, most patients had mild or less pre-discharge PVL (98.4%). These findings are similar to those observed in the FORWARD study which reported 98.1% of patients with mild or less AR. This present study, however, did not meet its secondary endpoint of no or trace AR at discharge (59.2%) as compared to the performance goal of 67.1% (p>0.99), which was based on the results of the FORWARD study that evaluated the earlier Evolut R valve. This is similar to a single-centre report from Germany comparing 148 Evolut R valve patients with 74 Evolut PRO valve patients18 but contrary to the findings of the smaller US Evolut PRO study, where initial experience in 60 patients demonstrated no/trace PVL in 72.4% of patients7. Possible reasons for this difference in this study may include the following. 1) Degree of annular calcification and distribution – the significantly larger and less selected cohort of patients would have included a more heterogeneous group of patients, with greater calcification burden, impacting adversely on the PVL observed. 2) More patients in the Forrest et al cohort underwent predilation as compared to this study (52% vs 34%), while the rate of post-dilation was similar (27% vs 31%) (perhaps predilation improves device contact with the surrounding anatomy and resultant seal). 3) Assessment of PVL/AR was extremely sensitive in the current study; the differentiation between trace and mild AR is difficult to make. The addition of an external pericardial wrap on the Evolut PRO valve did not adversely affect the new post-TAVI pacemaker rate of 20.7%. Previous generations of this device, by adhering to “best practice” to reduce conduction disturbances, have reported permanent pacemaker rates of 21.6% with the CoreValve® bioprosthesis (Medtronic)17 and 17.5% with the Evolut R valve14. The new PPI rate reported here could also be due to a large (30%) proportion of patients with pre-existing conduction disturbances.
Study limitations
FORWARD PRO is a real-world, contemporary standard practice study. Patient selection bias could have been introduced into the study as selection was based on local Heart Team evaluation. The utilisation of an independent echocardiographic core laboratory and independent CEC adjudication, however, ensured the unbiased verification of the echocardiographic data and clinical events.
Conclusions
The 30-day results of the FORWARD PRO study, evaluating the Evolut PRO valve, support its safety and clinical effectiveness in treating symptomatic patients with severe aortic stenosis at intermediate, high or greater risk for surgical aortic valve replacement. Longer-term follow-up will be required to evaluate these findings further.
|
Impact on daily practice Our analysis from the FORWARD PRO study reports a low rate of the primary endpoint of all-cause mortality (3.2%) with real-world use of the Evolut PRO valve, which can be resheathed and/or recaptured to aid in accurate valve positioning. This valve also has an outer pericardial wrap with the intention of reducing PVL. In our study, only 1.6% of patients had moderate or severe PVL at discharge. It is also recognised that the PVL rates observed with this genre of valve may improve with time. Longer-term follow-up data will help further evaluation of the safety and efficacy profiles and PVL rates of this novel device. |
Acknowledgements
Jane Moore, MS, ELS, drafted the methods and results, created all the tables and figures, and formatted the paper for journal style. Maarten Hollander, MSc, and Linda Schepers, MSc, from the Medtronic Bakken Research Center (Maastricht, the Netherlands), were responsible for overall study management. All are employees of Medtronic plc.
Funding
Medtronic (Minneapolis, MN, USA) funded the FORWARD PRO study.
Conflict of interest statement
G. Manoharan has served as a proctor for Medtronic and Abbott. E. Grube serves on an advisory board for Medtronic, Boston Scientific and HighLife, and has an equity interest in CardioValve, Valve Medical, Shockwave, Millipede, Pi-Cardia, Pipeline, Ancora and Laminar. N. Van Mieghem has received grant support from Abbott Vascular, Boston Scientific, Edwards Lifesciences and Medtronic, and advisory fees from Abbott, Boston Scientific, PulseCath BV and Medtronic. S. Brecker has received consultant fees from Medtronic, Boston Scientific and Meril Life Sciences. H. Danenberg is a clinical proctor for Medtronic. C. Tamburino reports personal fees from Medtronic during the conduct of the study and personal fees from Medtronic outside the submitted work. J.K. Oh has received research support for the echocardiographic core laboratory from Medtronic. Y. Fan is an employee and shareholder of Medtronic, plc. S. Windecker has received grant support from Abbott, Amgen, Bayer, Biotronik, BMS, Boston Scientific, Cardinal Health, CSL Behring, Daiichi Sankyo, Edwards Lifesciences, Johnson&Johnson, Medtronic, Polares Medical, Querbeet, Sanofi and Terumo outside the submitted work. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

