Abstract
Aims: To assess the relationship between a low preprocedural (<40 mmHg) mean transaortic gradient (MTG) and cardiovascular mortality following transcatheter aortic valve implantation (TAVI).
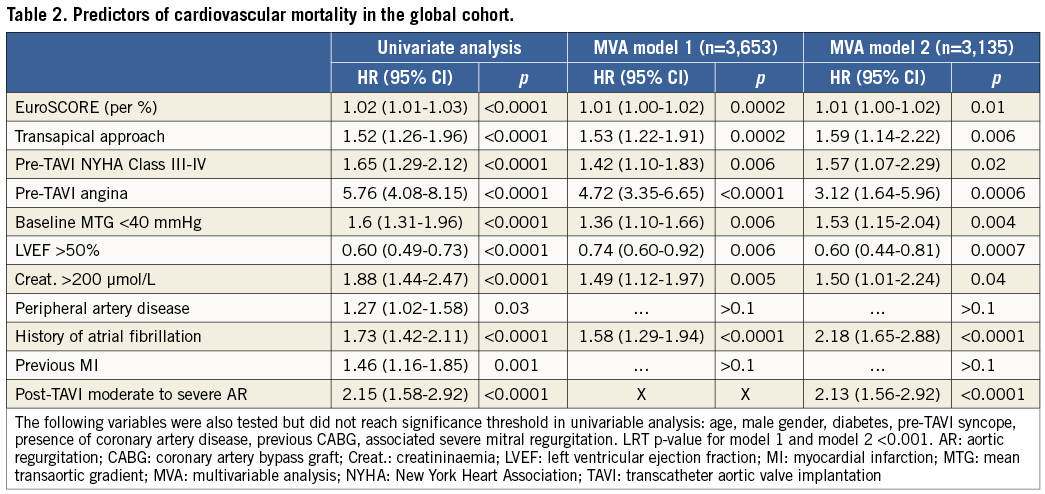
Methods and results: We studied highly symptomatic patients at high surgical risk who underwent TAVI, included in the FRANCE 2 multicentre registry. The primary endpoint was the incidence of any cardiovascular death in the year following the procedure. N=3,933 patients (age=82.8±7.2 years; EuroSCORE=21.8±14.1; left ventricular ejection fraction [LVEF]=55.5±12.6%) were enrolled. Low MTG was present in 23.5% of the cases. The one-year cardiovascular mortality was 13.3%. Cardiovascular survival was significantly lower in low MTG patients compared to the others. Multivariable Cox regression analysis revealed that a low MTG independently predicted cardiovascular death (HR=1.53 [1.15-2.04], p=0.004). Other independent predictors of cardiovascular mortality included preprocedural angina (HR=3.12 [1.64-5.96], p=0.0006); NYHA functional Class III-IV (HR=1.57 [1.07-2.29], p=0.02); severe renal failure (HR=1.50 [1.01-2.24], p=0.04); EuroSCORE (HR=1.01 [1.00-1.02], p=0.01); transapical access (HR=1.59 [1.14-2.22], p=0.006); impaired LVEF (HR=1.66 [1.23-2.27], p=0.0007) and post-procedural moderate to severe periprosthetic regurgitation (HR=2.13 [1.56-2.92], p<0.0001).
Conclusions: Presence of a low MTG prior to TAVI was associated with a greater risk of cardiovascular death up to one year following the procedure and could be used to identify patients at high risk for adverse cardiovascular outcomes following TAVI.
Introduction
Transcatheter aortic valve implantation (TAVI) is now recognised and recommended as an alternative for selected patients with severe aortic stenosis (AS), particularly for those at high surgical risk1. TAVI is associated with improved mortality rates, left ventricular (LV) haemodynamics and remodelling, NYHA functional class, and quality of life2. Although the outcomes are improving due to progress in the technique and a better selection of patients, fatal cardiovascular events still represent the primary cause of mortality3,4. The multicentre FRANCE 2 registry reported a 14.3% incidence of cardiovascular death at one year following the procedure, accounting for 60% of total deaths4. Compared to what is known about the risks associated with surgical aortic valve replacement (SAVR), data regarding the predictors of long-term outcome following TAVI are quite limited, due to the relative infancy of the technique. Furthermore, very few studies have focused specifically on the determinants of cardiovascular outcome.
A major risk factor of poor outcomes in AS is the presence of a low mean transvalvular gradient (MTG). Severe AS presenting with a low MTG is common not only in the setting of a reduced LV ejection fraction (LVEF)5 but also in patients with preserved LVEF. Accordingly, patients with low-gradient AS (LGAS) tend to experience worse outcomes compared to patients with a high transvalvular gradient (HGAS), treated either medically or surgically6-9. Previous reports have suggested that patients with a low MTG have worse outcomes following TAVI compared to the others10-12, but these data were limited by the sample size or endpoint definitions. In the light of these results, in the present study we investigated the impact of a low MTG, serving as a non-invasive measure of myocardial reserve, as an important clinical risk factor for adverse cardiovascular events following TAVI.
Material and methods
PATIENTS
This study prospectively included all patients who were included in the FRench Aortic National CoreValve and Edwards (FRANCE 2) registry between January 2010 and June 20124. Severe AS was defined as an indexed aortic valve area ≤0.6 cm2/m2, a mean aortic valve gradient ≥40 mmHg, or a peak aortic jet velocity ≥4.0 m/s4. All patients had New York Heart Association (NYHA) Class II, III, or IV symptoms. Criteria by which patients were deemed inappropriate for SAVR included: logistic EuroSCORE ≥20%; Society of Thoracic Surgeons Predicted Risk of Mortality (STS-PROM) ≥10%; and/or contraindications to surgery such as the presence of porcelain aorta, severe respiratory failure, and/or the presence of a prior left internal mammary artery (LIMA) bypass with unfavourable anatomy for a redo sternotomy.
ECHOCARDIOGRAPHIC ASSESSMENT
Echocardiographic images were acquired and interpreted in each centre by trained operators before and after the procedure. Evaluation of AS included: measurement of the MTG, maximal peak transvalvular velocity, and calculation of indexed aortic valve area (AVA) using the continuity equation. AS severity, aortic valve regurgitation and mitral regurgitation were graded according to international guidelines criteria previously described13. LVEF was assessed in all patients using the biplane Simpson’s method. LGAS was defined as the presence of both AVA <0.6 cm2/m2 and an MTG <40 mmHg at the time of the referral10,11.
TAVI PROCEDURE
Each multidisciplinary team (Heart Team) could choose to implant one of two commercially available valves: the balloon-expandable Edwards SAPIEN (or SAPIEN XT) prosthesis (Edwards Lifesciences, Irvine, CA, USA), or the self-expandable CoreValve (Medtronic, Minneapolis, MN, USA). The technical aspects of the TAVI procedure have been reported in detail previously4.
STUDY ENDPOINTS AND DATA MANAGEMENT
Following discharge from the index hospitalisation for TAVI, all patients were followed up through clinic visits and phone contact and were under surveillance for the VARC-2 updated consensus-defined adverse events14. Mortality was adjudicated by an independent clinical events committee. Database quality control was performed as previously reported4. The primary endpoint was defined as the occurrence of any cardiovascular death during the first year following the procedure.
STATISTICAL ANALYSES
Data are given as absolute numbers, percentages, and means (±standard deviation/SD). Continuous and categorical variables were compared using the Student’s t-test (for independent or paired samples) and chi-square or Fisher’s exact test, respectively.
Survival curves were constructed for time-to-event variables using Kaplan-Meier estimates and compared by log-rank test. Patients who were lost to follow-up were censored at the time of the last contact. Multivariable Cox models were used to assess the relation of clinical/echocardiographic covariates with the incidence of the primary endpoint within one year following the procedure. Univariate Cox proportional hazards regression analyses were performed first, then all covariates with a p-value of <0.1 were included in the multivariable regression model and backward stepwise elimination was performed to identify independent predictors of the primary endpoint. Two multivariable Cox regression models were used: the first model only included the baseline clinical and echographic characteristics, whereas the second one added post-TAVI periprosthetic regurgitation to these previous parameters. The validity of the proportionality assumption was verified by the likelihood ratio test (LRT). A two-sided alpha level of 0.05 was considered the threshold for statistical significance. All statistical analyses were performed using SAS statistical software version 9.3 (SAS Institute, Cary, NC, USA).
Results
BASELINE CHARACTERISTICS
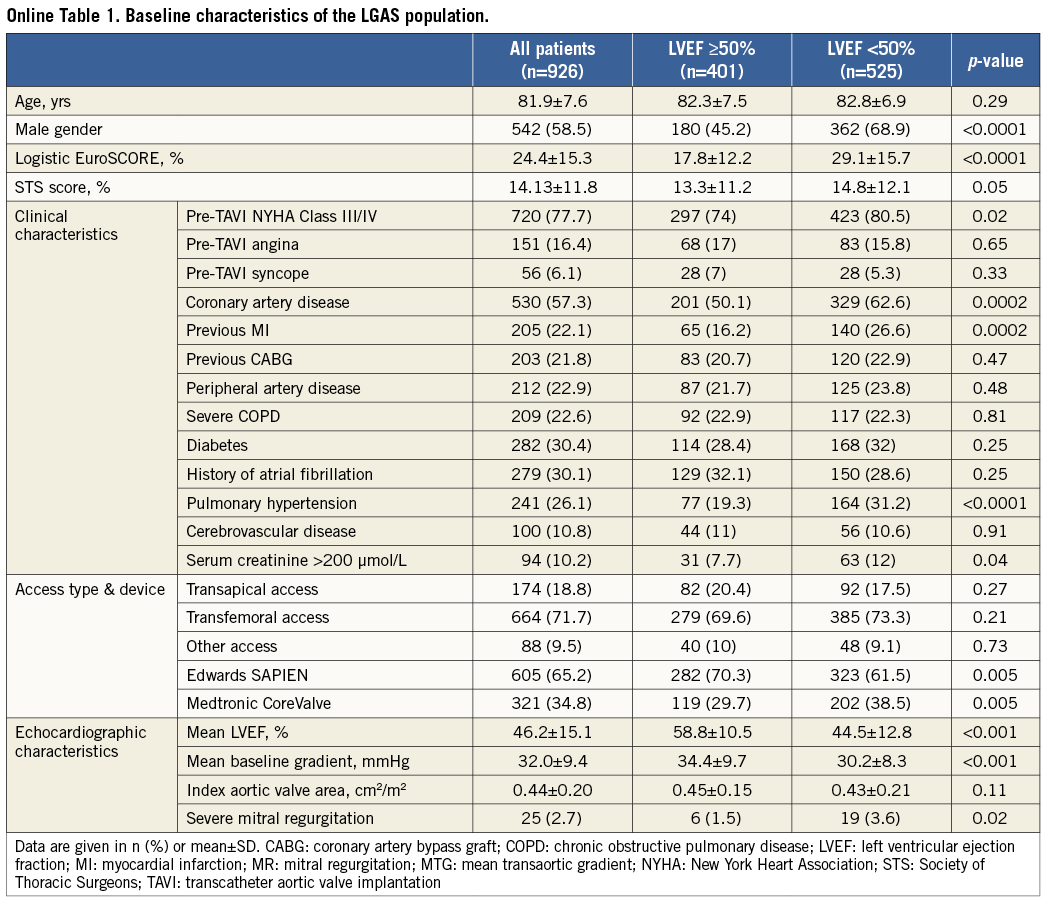
The baseline characteristics of the 3,933 consecutive patients included in the study are shown in Table 1. A low MTG was present in 23.5% of the patients. Among these patients, 525 (56.7%) had a baseline LVEF <50% (Online Table 1). Compared to patients with high preprocedural MTG (≥40 mmHg), patients with low baseline MTG (<40 mmHg) were more frequently men, and were younger, but had a higher logistic EuroSCORE and a higher number of associated comorbidities.
ENDPOINT ASSESSMENT
Procedural success was achieved in 97.1% of patients. Post-procedural aortic regurgitation was observed in 15.7% of the cases. Median follow-up was 204 days (interquartile range: 303), and was complete for 3,765 patients (95.7%). A total of 810 deaths were reported, including 430 cardiovascular deaths. Global and cardiovascular actuarial survival rates were, respectively, 90.3% and 92.8% at 30 days, whereas they were, respectively, 79.4% and 86.7% at one year.
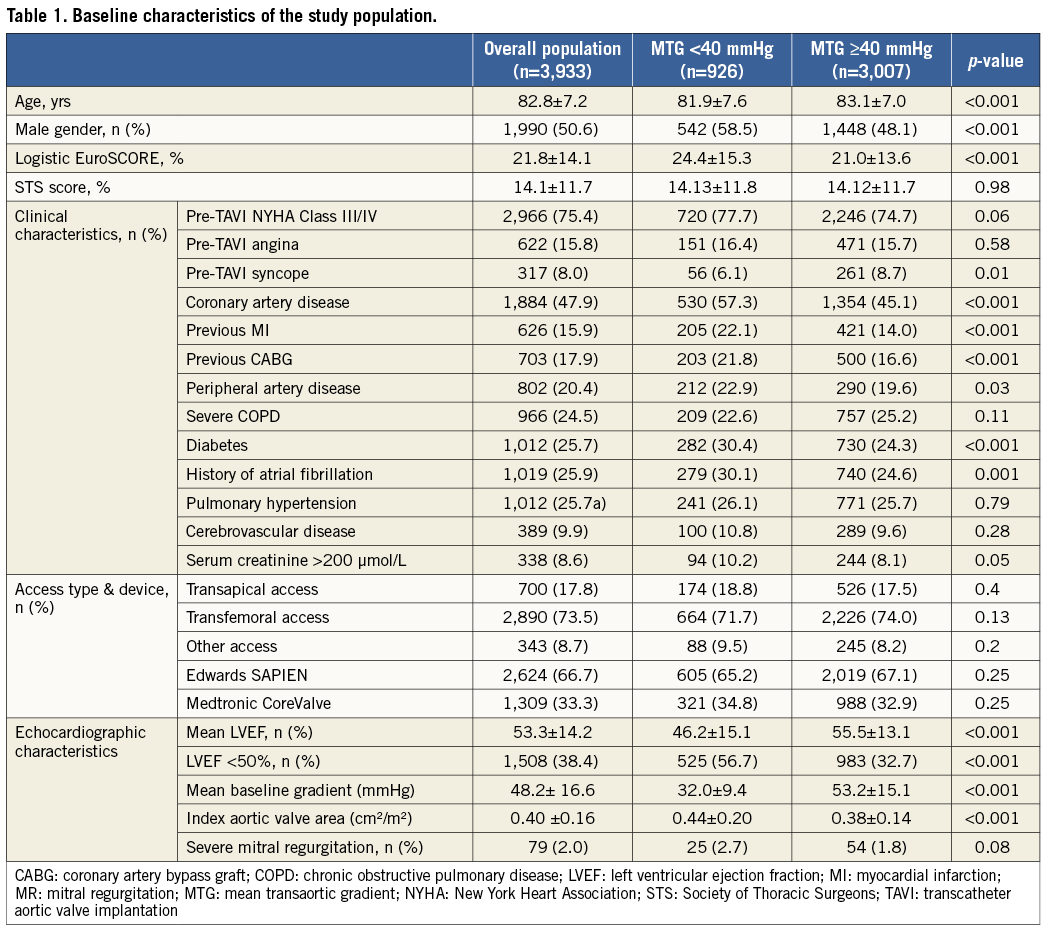
Early 30-day and one-year global survivals were significantly lower in patients with low versus high preprocedural MTG (respectively, 88.3% vs. 90.0% and 69.7% vs. 77.7%, p<0.001) (Figure 1A). As hypothesised, the 30-day and one-year cardiovascular survival rates were also reduced in patients with LGAS versus HGAS (respectively, 90.6% vs. 93.5% and 81.3% vs. 88.1%, p<0.001) (Figure 1B).
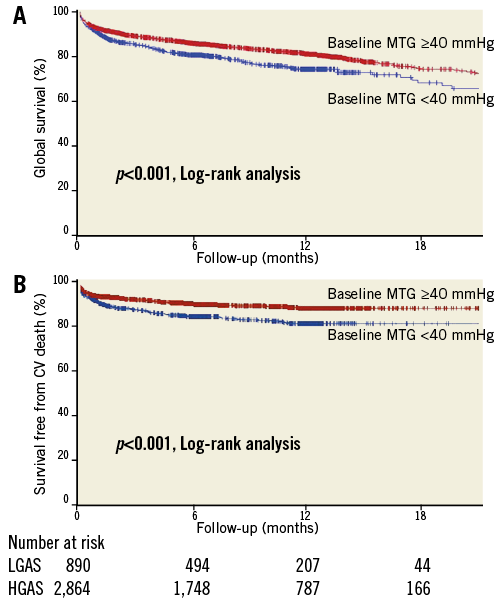
Figure 1. Global (A) and cardiovascular survival (B) in LGAS vs. HGAS patients.
In order to investigate further the respective roles of LGAS and impaired LV function, we stratified the patients into four groups according to their baseline LVEF and mean transaortic gradient. We observed that patients with both impaired LV function (LVEF <50%) and LGAS (Group 4/n=502) had a significantly higher mortality and a higher incidence of cardiovascular death compared to the others (Figure 2). The one-year actuarial cardiovascular survival was 77.2% for Group 4 patients vs. 89.7% for patients with LVEF ≥50% and MTG ≥40 mmHg (Group 1/n=1,936), 86.6% for patients with LVEF ≥50% and MTG <40 mmHg (Group 2/n=385), and 84.8% for patients with LVEF <50% and MTG ≥40 mmHg (Group 3/n=934).
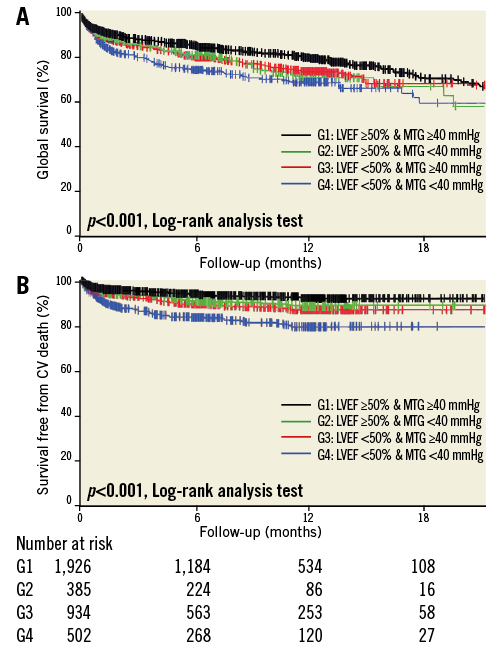
Figure 2. Global (A) and cardiovascular survival (B) according to baseline LVEF and MTG.
PREDICTORS OF CARDIOVASCULAR MORTALITY IN THE STUDY POPULATION
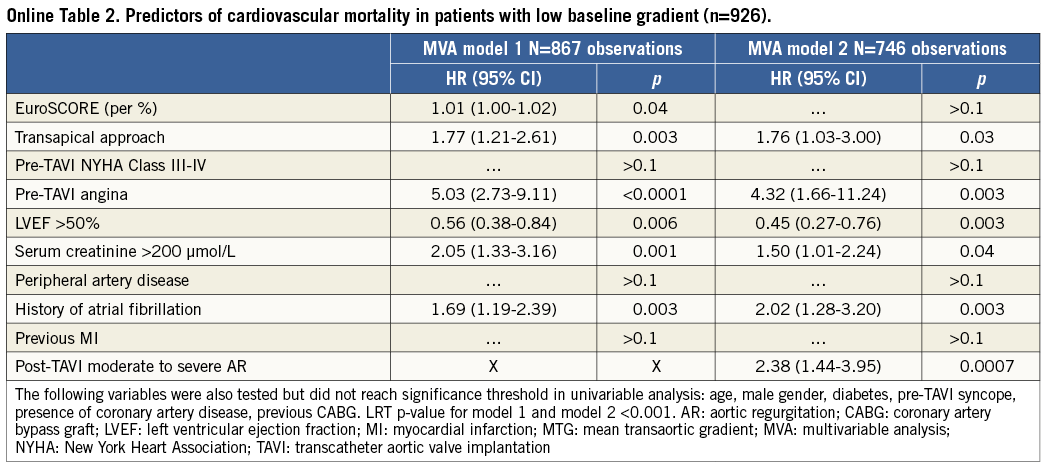
The results showed that low preprocedural MTG was an independent predictor of cardiovascular mortality, in addition to EuroSCORE, pre-TAVI angina, pre-TAVI NYHA functional Class III-IV, severe renal failure, impaired LVEF, transapical access, history of atrial fibrillation and post-intervention moderate to severe periprosthetic aortic regurgitation (Table 2). Interestingly, we observed no significant effect of previous myocardial infarction, previous coronary artery disease or previous CABG on cardiovascular survival.
VARIATIONS OF LVEF AND TRANSAORTIC GRADIENT ACCORDING TO BASELINE LVEF AND MTG
LVEF measurement six months after the initial procedure was available in 1,447 patients. LVEF increased significantly from 46.4±15.1 to 52.6±12.7% in LGAS patients (n=313) and from 56.2±13.1 to 59.3±11.5% in HGAS patients (n=1,126; p<0.0001 for both groups). At the same time, MTG decreased significantly in both groups (from 32.0±9.4 to 9.3±3.6 mmHg in LGAS patients, and from 53.2±15.1 to 10.8±5.3 mmHg in HGAS patients).
We then specifically analysed evolution in the four groups of patients according to their baseline MTG and LVEF. Although MTG significantly and uniformly decreased in all groups of patients, significant improvement of LVEF was only observed in patients with impaired baseline LV function, regardless of baseline MTG (Figure 3). The average LVEF increased from 42.9±10.5% to 53.3±12.4% in Group 3 after TAVI (average net increase: 10.4±12.4%), and from 36.3±9.4% to 47±11.7% in Group 4 (average net increase: 11.0±12.0%). Both baseline and six-month LVEF were significantly higher in Group 3 compared to Group 4 patients (p<0.001), but there was no statistical difference regarding the net increase (p=0.65).
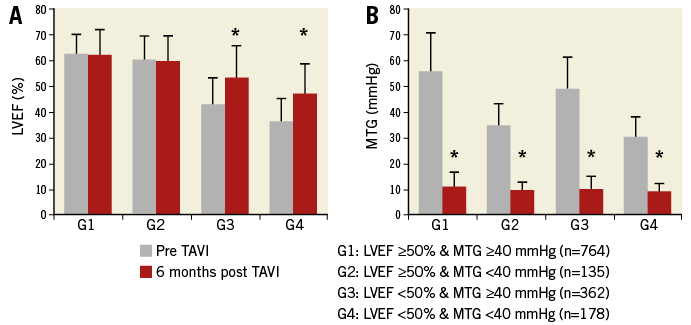
Figure 3. Evolution of LVEF and MTG before and six months after TAVI, according to baseline characteristics. * p<0.0001.
PREDICTORS OF CARDIOVASCULAR MORTALITY IN PATIENTS WITH LOW-GRADIENT AORTIC STENOSIS
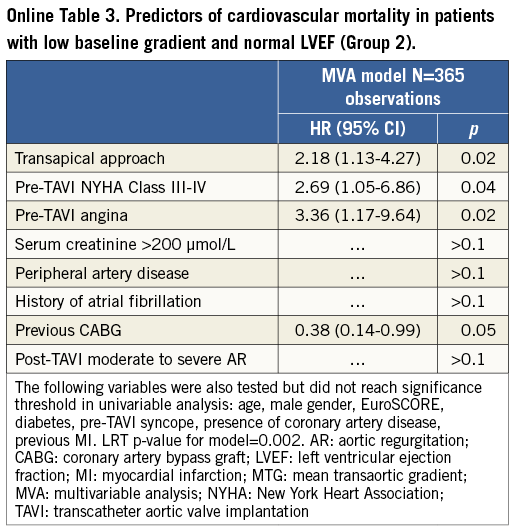
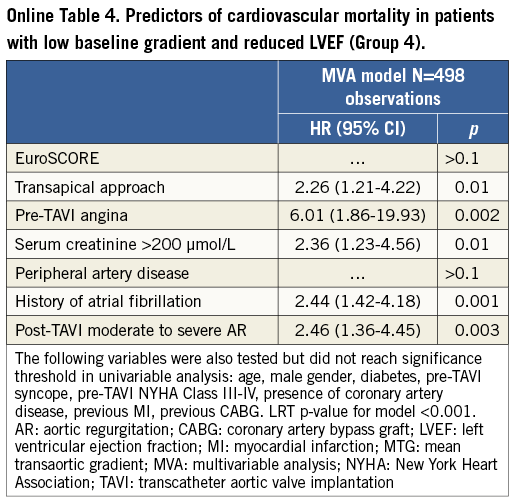
The factors associated with cardiovascular mortality in the LGAS patients (n=933) were: pre-TAVI angina, severe renal failure, baseline LVEF <50%, transapical access, history of atrial fibrillation and post-intervention moderate to severe periprosthetic aortic regurgitation (Online Table 2). Predictors of cardiovascular mortality in LGAS patients from Groups 2 and 4 are provided in Online Table 3 and Online Table 4.
Discussion
In the present study, we observed that the presence of a low baseline MTG was associated with an increased risk of cardiovascular death following TAVI, regardless of LVEF. These results: 1) identify new potential clinical and echographic markers for adverse cardiovascular outcome prediction following TAVI, and 2) highlight the significance of myocardial dysfunction and remodelling in patients with low-gradient/severe AS, even in the presence of preserved LVEF, as a source of additional risk.
The presence and the consequences of an MTG <40 mmHg in patients with severe aortic stenosis remains debated. This condition may be present in up to 30% of cases according to the published series15. In the context of severe AS, a low gradient reflects the existence of a low flow that can result from two different situations15. On the one hand, low flow can be related to gross systolic dysfunction manifesting with a reduced LVEF and/or afterload mismatch. On the other hand, it can be the consequence of reduced stroke volume despite a preserved LVEF. The latter situation has been described as “paradoxical low-flow aortic stenosis” or PLFAS16, which is reported to be present in up to 35% of patients with AS6. Baseline low gradient is a well-known predictive factor of adverse outcome in the evolution of severe AS treated either medically16 or by SAVR8. Although baseline reduced MTG is associated with higher surgical mortality, previous data have shown that aortic valve replacement could improve outcomes in these patients, regardless of the baseline LVEF and the presence of a contractile/flow reserve16,17. As a consequence of their high-risk profile due to associated comorbidities, these patients are frequently considered unsuitable for conventional surgery, and thus treated medically. Hence, TAVI could offer an appropriate management option in this situation15.
Recent studies have investigated the feasibility and impact of LGAS on outcome following TAVI in these patients10,11,18-20. In these series, LGAS was associated with a higher risk profile compared to other patients10,11,18-20. According to the data obtained from SAVR, this condition was associated with a worse early and midterm outcome10-12,18,19. Our series is in line with these results, since we observed a higher incidence of associated comorbidities and increased global and cardiovascular mortality in LGAS patients compared to HGAS subjects, highlighting the independent role of baseline low aortic gradient on outcomes as previously observed10,18,19. Regarding the large size of the present cohort, we were also able to identify the predictors of outcome in the specific LGAS group and to analyse LVEF evolution following TAVI in this subgroup of patients, showing that LV function can improve over time even in case of low baseline MTG.
The factors contributing to the adverse impact of a low gradient on outcome have not been clarified, but might be related to underlying myocardial abnormalities in these patients21. The natural history of AS is characterised by an increase in the pressure afterload on the LV, inducing a hypertrophic response with increased wall thickness and enlarged cardiomyocytes21. Although this response restores wall stress initially, the persistent valvular abnormality will subsequently induce some maladaptive phenomena including increased muscular fibrosis, cellular apoptosis and abnormal angiogenic response with decreased capillary density22. These phenotypic changes are considered to precede the transition from normal to impaired left ventricular function, could lead to impaired myocardial microcirculation and might expose patients to ischaemic complications22. Given this perspective, the existence of a pre-intervention angina could bear witness to a more advanced myocardial disease, explaining the intriguing relationship we observed between this clinical parameter and adverse outcome, regardless of the presence of an underlying coronary artery disease. Moreover, the existence of LGAS is related to decreased flow through the narrowed valve and probably reflects the presence of these advanced myocardial alterations.
Recently, Herrmann and colleagues observed that patients with LGAS had more advanced myocardial fibrotic lesions and impaired left ventricle contraction than patients with a normal gradient23. Collectively, these data suggest that a low baseline gradient reflects the presence of severe myocardial abnormalities during the evolution of AS, which can account for the increased cardiovascular deaths observed in this subgroup of patients. Similar to SAVR, the TAVI procedure promptly corrects AS and dramatically decreases LV afterload in the short term. However, recovery of LV function and reverse remodelling are more progressive processes, and patients could be overexposed to complications during this myocardial “healing” phase. Interestingly, we observed that patients with impaired baseline left ventricular function had a dramatic increase in LVEF, despite higher mortality compared to the other groups, which confirms that these patients derive a great benefit from the procedure24.
Limitations
Although this study presents the largest cohort of patients with LGAS who underwent TAVI, it has several limitations that warrant consideration. These data are based on a multicentre registry and there was no echocardiography core lab to reanalyse the measurements. We were not able to analyse the values of LV stroke volume (SV), as it was not collected in the database, and were thus unable to identify the patients with low-flow aortic stenosis according to the recognised criteria16. Consequently, we do not know if these subgroups of subjects had different outcomes as suggested by recent data18,19. However, these results (as well as ours) are based on echocardiographic gradient assessment. Recent reports have highlighted the discrepancies which might exist between invasive and non-invasive evaluation of aortic stenosis in elderly AS patients25,26, suggesting that non-invasive SV measurement might not be reliable in selected patients, due to left ventricle outflow tract geometry modifications and variations in the post-load conditions25,26. Thus, the use of SV measurement as a stratification tool for outcome prediction could be questioned in this situation. Furthermore, data regarding the results of dobutamine stress test in case of LGAS with reduced LVEF were not routinely collected in the national database, which precluded us from distinguishing primary myocardial dysfunction from afterload mismatch cases. Finally, the data in the present report are limited to the one-year results. We need a longer clinical follow-up to assess whether the long-term evolution is different among the different patient groups.
Conclusions
In conclusion, our data indicate that the cardiovascular prognosis of patients undergoing TAVI is influenced by the preprocedural MTG in addition to other clinical and procedural factors. Thus, the value of baseline MTG may represent a simple marker for identifying patients who are at particularly high risk of developing adverse cardiovascular events following TAVI.
| Impact on daily practice Measurement of preprocedural transaortic gradient (MTG) represents a simple way to assess the left ventricle performance in patients with aortic valve stenosis. Low MTG (<40 mmHg) is associated with increased cardiovascular mortality following transcatheter aortic valve implantation (TAVI), independent of baseline left ventricle ejection fraction and in addition to other known prognostic factors. Thus, baseline MTG may represent a simple marker for identifying patients who are at particularly high risk for developing adverse cardiovascular events following TAVI. |
Funding
The FRANCE 2 registry was funded by a research grant from Edwards Lifesciences and Medtronic.
Conflict of interest statement
N. Amabile has received travel support from Edwards Lifesciences. H. Eltchaninoff has received consulting fees and travel support from Edwards Lifesciences. C. Caussin has received consulting and proctoring fees from Edwards Lifesciences. The other authors have no conflicts of interest to declare.