Abstract
Abstract: Over the past decades, percutaneous coronary intervention (PCI) has become the most common modality for myocardial revascularisation, and it is increasingly used in patients with advanced coronary artery disease. Antithrombotic therapy, including antiplatelet and anticoagulant drugs, plays a key role and should be part of the optimal revascularisation strategy in the early phase as well as in the long-term prevention of ischaemic events. An antithrombotic therapy regimen of increased intensity and/or duration may mitigate part of the ischaemic burden associated with complex PCI. However, patients undergoing complex PCI are often at increased bleeding risk, challenging, therefore, the decision-making process. In this setting, the optimal antithrombotic treatment is still a matter of debate and has become a field of intensive research. In this state-of-the-art review, we analyse the evidence related to the different approaches regarding the periprocedural and long-term antithrombotic management of patients undergoing complex PCI. Since a “one-size-fits-all” approach cannot be justified in this clinical setting, our aim is to tailor the antithrombotic strategy to each patient’s profile and PCI complexity. We discuss the type and duration of antithrombotic regimens that can be selected for patients undergoing complex PCI, with a focus on prolonged dual antiplatelet therapy, P2Y12 receptor inhibitor monotherapy, and dual pathway inhibition. We also address antithrombotic management in specific scenarios (left main disease, coronary bifurcations, chronic total occlusion) and in patients undergoing complex PCI who require oral anticoagulant therapy.
In approximately 30% of cases, percutaneous coronary intervention (PCI) features a certain level of technical or anatomical complexity1. Antithrombotic therapy in the setting of PCI has the following aims: (i) to minimise thrombus burden and prevent the no-reflow phenomenon in patients with acute coronary syndrome (ACS); (ii) to reduce thrombotic complications secondary to the mechanical damage derived from coronary intervention (such as distal embolisation, plaque rupture, iatrogenic dissection, and occlusion of side branches); (iii) to prevent stent thrombosis; and (iv) to prevent ischaemic events outside the stented coronary segments23.
In this state-of-the-art review, we summarise the currently available antithrombotic strategies in patients undergoing complex PCI and highlight the results of randomised clinical trials that have tested antithrombotic regimens in this setting (Figure 1).
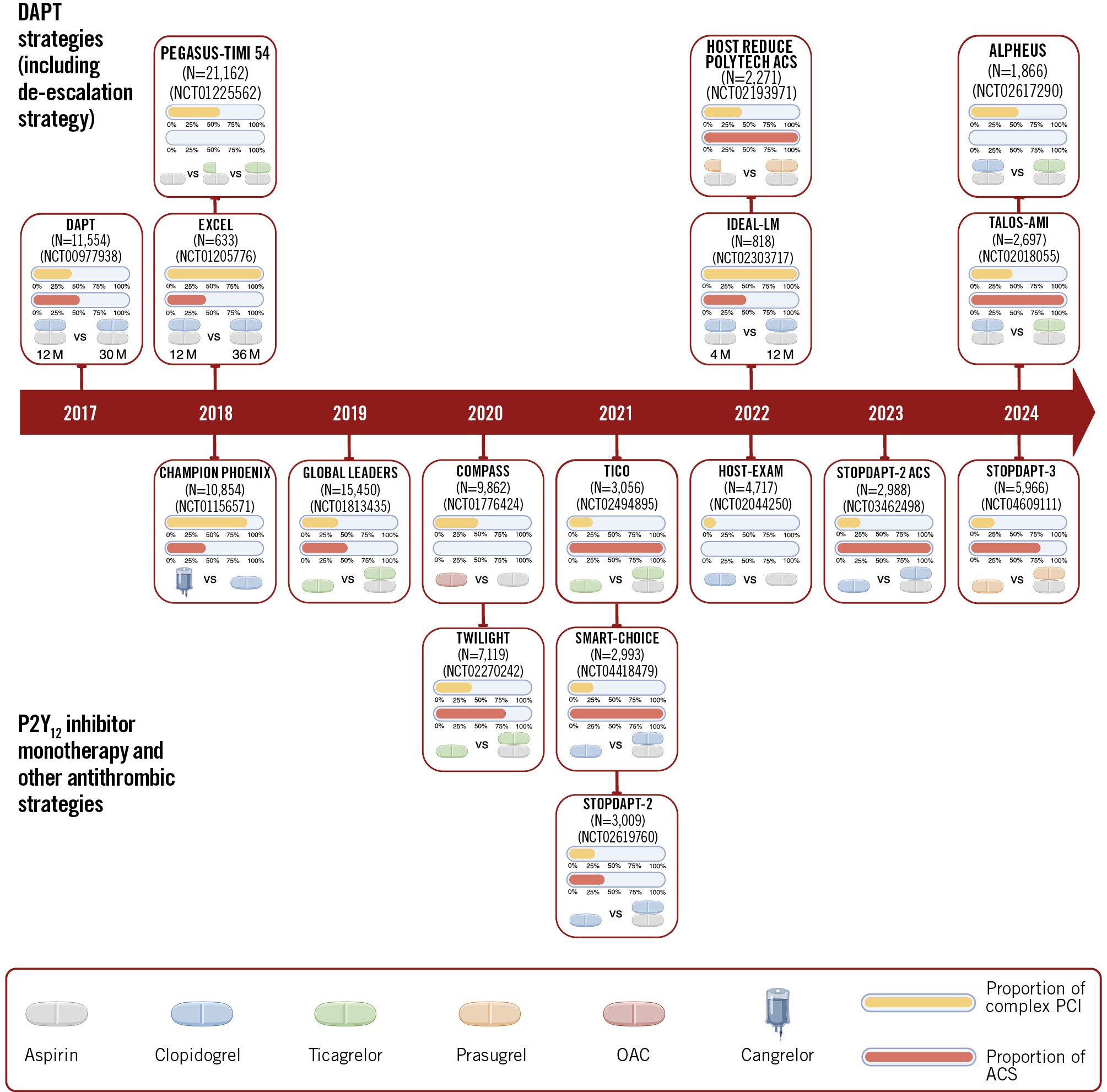
Figure 1. Timeline of randomised trials testing antithrombotic strategies in complex percutaneous coronary intervention. The duration is shown only for trials comparing short-term versus long-term DAPT. The publication year refers to the substudies performed in a complex PCI setting. ACS: acute coronary syndrome; DAPT: dual antiplatelet therapy; OAC: oral anticoagulant; PCI: percutaneous coronary intervention
Methods
We searched MEDLINE (from its inception to October 2024). We used the search terms “complex” or “complexity” in combination with the terms “percutaneous coronary intervention” or “PCI” or “revascularisation”. We selected publications from the past 10 years but did not exclude commonly referenced and highly regarded older publications. We also searched the reference lists of articles identified by this search strategy and selected those we judged relevant. Review articles are cited to provide readers with more details.
Complex PCI
Despite the lack of a universal definition, criteria for complex PCI generally include one of the following characteristics: treatment of 3 vessels or ≥3 lesions, implantation of ≥3 stents, treatment of bifurcation with a 2-stent technique, total stent length ≥60 mm, or a chronic total occlusion (CTO) as the target lesion4. Additional criteria, such as treatment of the left main (LM) or a saphenous vein graft (SVG), lesion length ≥30 mm, thrombus-containing lesions, the use of an atherectomy device for severely calcified lesions, or bifurcation lesions with a side branch diameter ≥2.5 mm, have also been reported (Figure 2)56. The concept of complex PCI differs from complex high-risk indicated PCI (CHIP-PCI), as the latter considers patient-related factors (e.g., elderly status, frailty, severe left ventricular dysfunction, or chronic kidney disease) and procedure-related factors (e.g., mechanical circulatory support)7.
Patients undergoing complex PCI tend to have more comorbidities and incur a higher risk of both early and late ischaemic events compared to those undergoing non-complex PCI, particularly in the presence of multiple complexity features124689. Factors such as longer procedural time, more extensive coronary manipulation, the presence of severe calcifications that hinder proper stent expansion, and an increased incidence of periprocedural complications contribute to an elevated early ischaemic risk. Conversely, incomplete revascularisation, the higher likelihood of stent failure, and accelerated disease progression play a greater role in late ischaemic events. Despite potentially being effective, antithrombotic therapy is invariably associated with an increased bleeding risk, which might override the ischaemic risk in certain populations510. Notably, major bleeding complications may portend a similar prognostic impact compared with thrombotic events11. While the link between complex PCI and ischaemic events is generally recognised, the impact of complex PCI on bleeding events is more controversial41213. Since antithrombotic therapy can mitigate the risk of both stent-related and non-stent-related events, the selection of an antithrombotic regimen based on its efficacy and safety profiles may significantly influence the outcomes of patients undergoing complex PCI. Although recommendations on alternative antithrombotic regimens in current European Society of Cardiology (ESC) guidelines refer to patients with high ischaemic risk, several technical aspects are potential criteria for complex PCI (≥3 stents implanted, treatment of ≥3 lesions, total stent length >60 mm, LM PCI, treatment of bifurcation with a 2-stent technique, CTO PCI)1415.
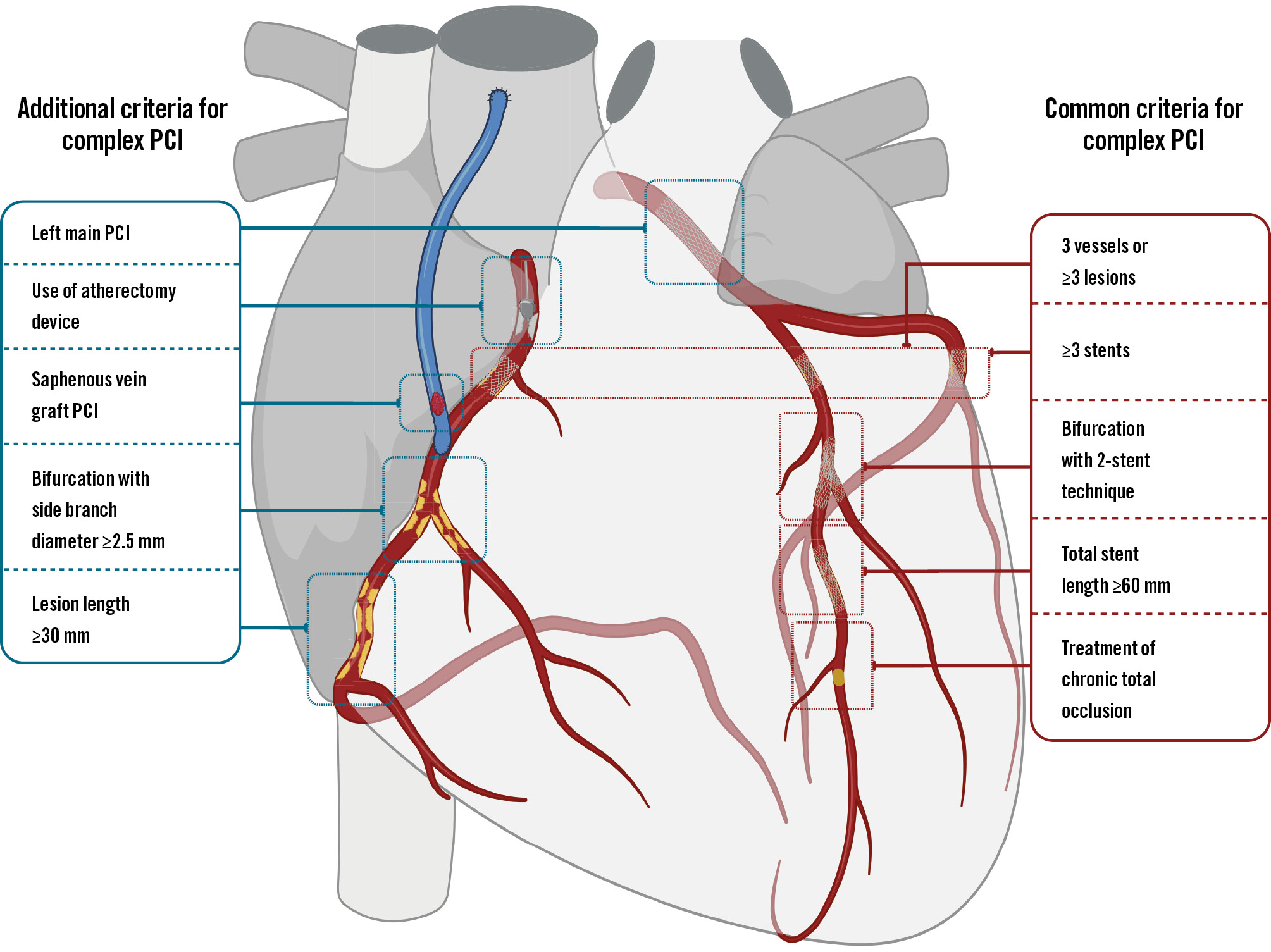
Figure 2. Principal and additional criteria for complex percutaneous coronary intervention. PCI: percutaneous coronary intervention
Antithrombotic therapy before PCI
In patients with unknown coronary anatomy, anticipating complex PCI is highly unlikely. However, not all patients undergo ad hoc PCI, and in several instances the decision to proceed with PCI is delayed (e.g., after Heart Team evaluation). In patients with chronic coronary syndrome (CCS), the prior recommendation to pretreat with clopidogrel for at least 5 days is no longer present in the current ESC guidelines on CCS1415161718. From a clinical standpoint, in patients scheduled for complex PCI and deemed at low bleeding risk based on their risk score (e.g., Academic Research Consortium for High Bleeding Risk [ARC-HBR])10 and procedural aspects (e.g., radial access), pretreatment is a valuable option. In other cases, a loading dose of P2Y12 inhibitors can be safely administered around the time of PCI141518.
In the setting of ACS, routine pretreatment with P2Y12 inhibitors is no longer recommended for patients with non-ST-segment elevation ACS (NSTE-ACS) undergoing early invasive assessment within 24 hours (Class III, Level of Evidence A) and is weakly recommended for ST-segment elevation myocardial infarction (STEMI; Class IIb, Level of Evidence B)14.
An intravenous bolus of unfractionated heparin (UFH) is recommended at the time of diagnosis in patients with STEMI (Class I, Level of Evidence C)14. Data from observational studies showed that pretreatment with UFH in patients with STEMI is associated with higher patency of the infarct-related artery at baseline angiography1920, and these data have been recently confirmed by a randomised trial21.
In patients with NSTE-ACS, fondaparinux is recommended when an invasive strategy within 24 hours is not anticipated (Class I, Level of Evidence B)14.
Antithrombotic therapy during PCI
Periprocedural antithrombotic therapy is mandatory during PCI to avoid thrombus formation on the surface of the intravascular equipment and at the site of local plaque rupture or dissection caused by balloon angioplasty and stent implantation2. Complex PCI is linked to a heightened risk of bleeding at the access site, particularly when using femoral access, larger sheath sizes, or mechanical circulatory support devices. Hence, determining the ideal periprocedural antithrombotic approach remains a subject of ongoing debate.
1. Anticoagulant therapy
Parenteral anticoagulant therapy with intravenous UFH is the standard of care in patients undergoing PCI by virtue of its low cost, the immediate onset of action, the ease of periprocedural monitoring using activated clotting time (ACT) with the opportunity to titrate the infusion in order to deepen the antithrombotic effect, and the possibility of being rapidly antagonisable by protamine sulphate22. Bivalirudin and intravenous enoxaparin are two alternatives to UFH, based on data from randomised trials showing a decreased risk of major bleeding232425. The rapid onset and stable antithrombotic effect of bivalirudin, with no need for repeated monitoring, are significant advantages for the use of bivalirudin in long, technically complex coronary procedures, even though the absence of a selective antagonist remains a concern for its use. Data on bivalirudin are more controversial in view of the increased risk of stent thrombosis and myocardial infarction (MI) reported by early trials26272829, findings not confirmed by the three most recent trials303132. Evidence interpretation about bivalirudin is also challenged by the variable use of glycoprotein IIb/IIIa inhibitors (GPI) in the control arms and the non-standardised scheme of bivalirudin infusion after PCI. Considering the shorter half-life of bivalirudin (25 min) compared with heparin (60-90 min), continuing a full dose of bivalirudin infusion for up to 2-4 h post-PCI may mitigate the early increased ischaemic risk after PCI, mainly driven by stent thrombosis, observed in previous trials. This hypothesis, initially supported by a post hoc analysis of the EUROMAX trial33, was recently confirmed in a patient-level meta-analysis of six randomised trials with 15,254 patients34. Bivalirudin reduced the risk of all-cause mortality, cardiac mortality and major bleeding, although with an increase in reinfarction and stent thrombosis compared with UFH. However, in four trials with a 2- to 4-hour high-dose post-PCI infusion regimen, bivalirudin reduced cardiac mortality and major bleeding, without increasing the risk of reinfarction or stent thrombosis34. Of interest, a prolonged infusion of bivalirudin after PCI reduced procedural myocardial injury severity without increasing the risk of bleeding in patients with CCS in the Safety and Efficacy of Prolonged Use of Bivalirudin 4 Hours After elective PCI (COBER) study35. In approximately 20% of patients included in the CHAMPION PHOENIX trial, bivalirudin was used as the anticoagulant during PCI. Of interest, the decreased risk of ischaemic complications without bleeding liability was retained in patients receiving cangrelor and bivalirudin36. Hence, even if there is still a lack of data in patients undergoing complex PCI, bivalirudin might counterbalance the possibly higher risk of bleeding associated with the use of more potent P2Y12 inhibitors and cangrelor.
Despite the lack of data regarding complex PCI, in a large meta-analysis of 23 randomised and non-randomised trials including 30,966 patients, enoxaparin (both intravenous and subcutaneous) significantly reduced mortality, MI, and the composite endpoint of death or MI compared with UFH, particularly in patients with STEMI undergoing primary PCI. Of note, major bleeding was reduced only with the intravenous route, a finding not observed in studies that used subcutaneous enoxaparin25.
To summarise, although there are no specific data regarding the optimal periprocedural anticoagulant management in the complex PCI setting, UFH is widely used and remains the default strategy for anticoagulant therapy. However, bivalirudin and enoxaparin represent valuable alternatives for complex PCI, especially in patients at high risk of periprocedural bleeding events (Central illustration).
However, when UFH is used, in some complex procedures (e.g., retrograde CTO PCI), achieving a higher ACT (>350 seconds) may be considered37. The results of several studies on ACT monitoring during PCI are conflicting. A post hoc analysis of the Treatment of Acute Coronary Syndromes With Otamixaban (TAO) trial represents, to date, the largest cohort with systematic blinded ACT monitoring38. In this cohort of patients with NSTE-ACS treated with UFH plus GPI undergoing PCI, there was no evidence of an ACT threshold predicting ischaemic complications, whereas peak procedural ACTs ≥250 seconds for femoral access and ≥290 seconds by radial approach were associated with increased bleeding risk38.
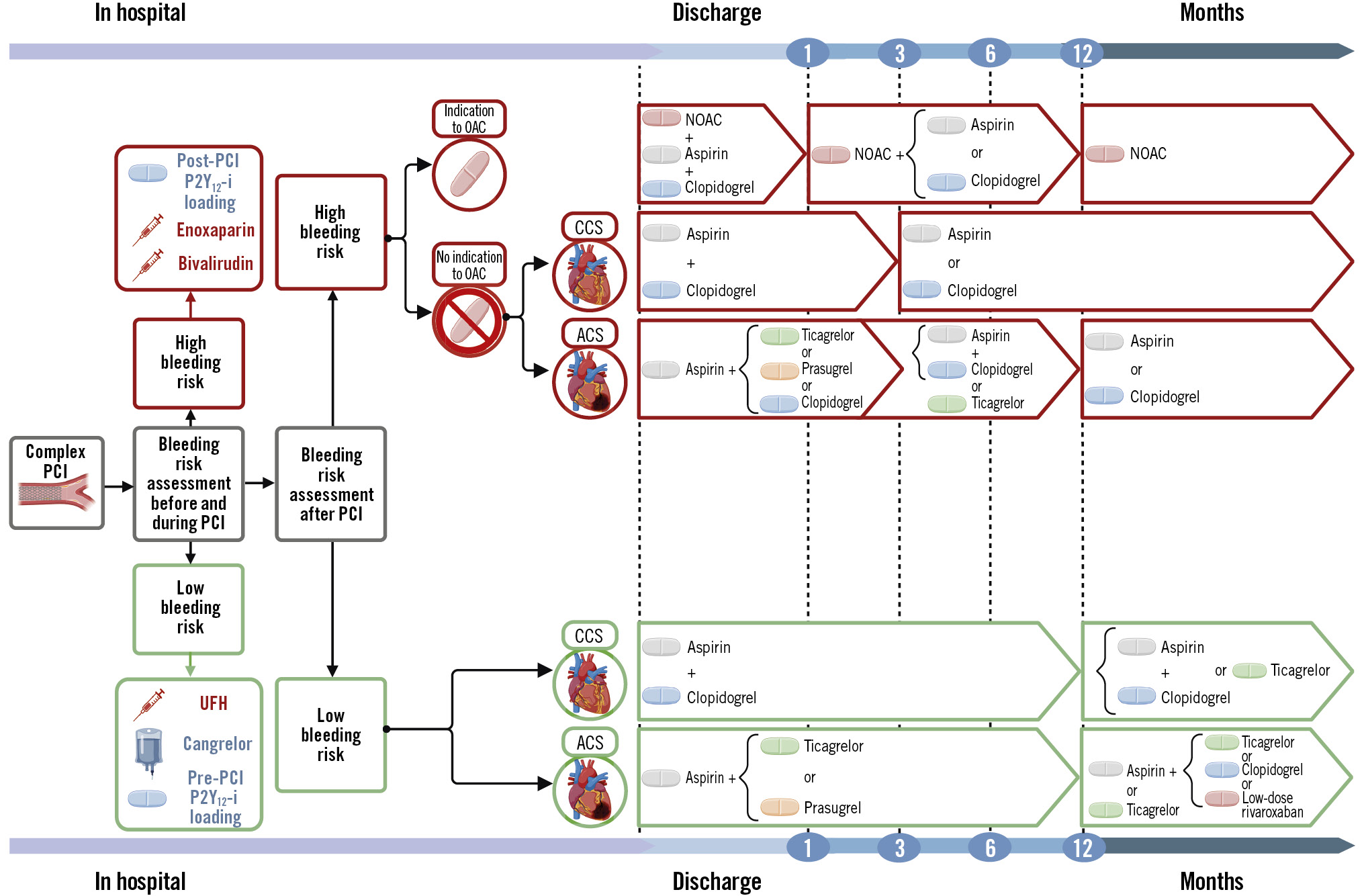
Central illustration. Antithrombotic strategies for patients undergoing complex percutaneous coronary intervention. The algorithm shows periprocedural and long-term antithrombotic strategies based on bleeding risk. All patients undergoing complex PCI are assumed to be at high ischaemic risk. Patients requiring OACs are assumed by default to be at HBR. ACS: acute coronary syndrome; CCS: chronic coronary syndrome; HBR: high bleeding risk; NOAC: non-vitamin K antagonist oral anticoagulant; OAC: oral anticoagulant; P2Y12-i: P2Y12 inhibitor; PCI: percutaneous coronary intervention; UFH: unfractionated heparin
2. Antiplatelet therapy
Periprocedural aspirin use, along with P2Y12 inhibitor administration, is the mainstay in patients undergoing PCI and is also standard in complex PCI. Clopidogrel is usually the default P2Y12 inhibitor in the setting of elective PCI, whereas ticagrelor or prasugrel are preferred in patients with ACS and may be considered in patients with CCS at high ischaemic risk (Class IIb, Level of Evidence C)141518. Clopidogrel has been extensively used and remains the P2Y12 inhibitor of choice in patients with CCS, even in complex PCI1539, although its use is limited by delayed antiplatelet efficacy and high variability in the antiplatelet effect. As of yet, there is no reason to replace clopidogrel with more potent P2Y12 inhibitors to prevent periprocedural events in patients undergoing elective, complex PCI. Two randomised trials, Ticagrelor versus clopidogrel in elective percutaneous coronary intervention (ALPHEUS) and Strategies of Loading With Prasugrel Versus Clopidogrel in PCI-Treated Biomarker Negative Angina (SASSICAIA), failed to demonstrate the superiority of more potent P2Y12 inhibition using ticagrelor or prasugrel, respectively, compared with clopidogrel. These trials aimed to decrease periprocedural and cardiovascular events in patients with CCS undergoing high-risk PCI4041. Consistently, in the ALPHEUS substudy, randomisation to ticagrelor versus clopidogrel did not decrease periprocedural or 30-day cardiovascular events in patients with CCS undergoing complex PCI42.
Cangrelor is an intravenous P2Y12 inhibitor that acts as a direct reversible P2Y12 receptor antagonist, with rapid onset and offset after infusion discontinuation36. It is currently approved (Class IIb, Level of Evidence A) for oral P2Y12 inhibitor-naïve patients presenting with ACS undergoing PCI14. Compared to clopidogrel, cangrelor achieves faster and more potent platelet inhibition, and this finding has been confirmed in patients undergoing complex PCI43. In a pooled analysis of three trials, including 24,910 patients, cangrelor significantly reduced the risk of early ischaemic events at 48 hours after PCI compared with clopidogrel44. Although there was no significant difference in terms of the primary safety endpoint, the risk of major bleeding based on other criteria was significantly increased in patients randomised to cangrelor. Because the risk of periprocedural MI is significantly higher in patients undergoing complex compared to non-complex PCI, even using multiple definitions8, cangrelor may represent a valid alternative in patients naïve to P2Y12 inhibitors who are not at high bleeding risk (HBR). In this respect, the greatest absolute risk reduction in ischaemic events with cangrelor has been reported in patients with the highest number of high-risk features45.
GPI are potent intravenous antiplatelet agents, which were routinely added to UFH in patients with ACS in the early era of PCI. However, in view of the increased risk of major bleeding, GPI are currently indicated only as a bailout strategy in case of no-reflow or thrombotic complications during PCI. Recent ESC guidelines on ACS indicate the potential use of GPI in patients undergoing high-risk PCI and naïve to P2Y12 inhibitors14.
Antithrombotic therapy after PCI
1. Dual antiplatelet therapy
In patients undergoing PCI, a variable duration of dual antiplatelet therapy (DAPT), consisting of a combination of aspirin and a P2Y12 inhibitor, is recommended for all patients and still remains the cornerstone of secondary prevention.
In real-world clinical practice, 20-40% of patients continue DAPT beyond 1 year after revascularisation, and complex PCI is a major independent predictor of DAPT persistence46. In a patient-level meta-analysis including nearly 9,500 patients from 6 randomised trials, long-term DAPT (≥12 months), compared with short-term DAPT (3-6 months), yielded a significant reduction of major adverse cardiovascular events in the complex PCI group, a finding not observed in the non-complex PCI stratum (p for interaction=0.01). Moreover, the benefit associated with long-term DAPT gradually increased with the number of complex PCI features. However, long-term DAPT was associated with a significantly higher risk of major bleeding, both in complex PCI and in non-complex PCI strata4. A subsequent meta-analysis combined the risk of bleeding with PCI complexity in 8 trials and showed that in patients undergoing complex PCI without HBR features, prolonging DAPT (12 or 24 months) was associated with a decreased absolute risk of ischaemic events without bleeding liability, compared with short DAPT (3 or 6 months). In patients undergoing complex PCI and at HBR, prolonging DAPT increased the risk of bleeding without a parallel ischaemic benefit13. In a post hoc analysis of the DAPT trial, long-term DAPT (12-30 months), mainly with clopidogrel and prasugrel, was associated with a reduction of MI and stent thrombosis rates at the expense of an increased bleeding rate, a finding that was similar for patients with and without anatomical complexity6. Consistently, in a prespecified subanalysis of the Prevention of Cardiovascular Events in Patients with Prior Heart Attack Using Ticagrelor Compared to Placebo on a Background of Aspirin-Thrombolysis In Myocardial Infarction 54 (PEGASUS-TIMI 54) trial, extended DAPT with ticagrelor significantly reduced the risk of cardiovascular death, MI, or stroke, whereas it increased the risk of major bleeding, as compared to aspirin plus placebo, irrespective of multivessel coronary artery disease angiographic evidence47. Taken together, these data (Table 1) support a strategy of DAPT prolongation beyond 1 year after PCI in patients undergoing complex PCI when the thrombotic risk is prevalent (e.g., patients with ACS) and the bleeding risk is low (Central illustration). Extended DAPT (beyond 12 months) should be considered in patients without HBR and with high ischaemic risk, including those presenting with complex PCI (Class IIa, Level of Evidence A) according to current ESC guidelines on the management of ACS and CCS1415.
Table 1. Studies comparing short-term versus long-term DAPT strategies in patients undergoing complex PCI.
| Study - year | Complex PCI patients | ACS patients | Follow-up | Complex PCI criteria | Short-term DAPT | Long-term DAPT |
Results |
|---|---|---|---|---|---|---|---|
| Patient-level meta-analysis of 6 RCTs4 -2016 | 17.5%(1,680/9,577) | 43.7%(4,189/9,577) | 12 months* | ≥1 of the following: | 3 to 6 months of DAPT (aspirin and clopidogrel) | ≥12 months of DAPT (aspirin and clopidogrel) | Long-term DAPT yielded a significant reduction in MACE in the complex PCI group versus the non-complex PCI group |
| 3 vessels treated, | |||||||
≥3 stents implanted, |
|||||||
≥3 lesions treated, |
|||||||
bifurcation with 2 stents implanted, |
|||||||
total stent length >60 mm, |
|||||||
| CTO PCI | |||||||
| DAPTtrial6-2017 | 32.2%(3,730/11,554) | 46.3%(5,359/11,554) | 30 months | ≥1 of the following: | 12 months of DAPT (aspirin and clopidogrel or prasugrel) | 30 months of DAPT (aspirin and clopidogrel or prasugrel) | Long-term DAPT reduced ischaemic events, irrespective of the presence of anatomically complex lesions, but increased moderate/severe bleeding |
| unprotected LM, | |||||||
≥2 lesions per vessel, |
|||||||
lesion length ≥30 mm, |
|||||||
bifurcation lesion with side branch ≥2.5 mm, |
|||||||
saphenous vein graft, |
|||||||
thrombotic lesions |
|||||||
| EXCEL trial-2018 | 100%(633/633) | 38.7%(245/633) | 36 months | LM PCI | 12 months of DAPT (aspirin and clopidogrel, prasugrel or ticagrelor) | 36 months of DAPT (aspirin and clopidogrel or ticagrelor) | Long-term DAPT was not associated with improved event-free survival in complex PCI patients |
| PEGASUS-TIMI 54 trial4786-2018 | 59.4%(12,558/21,162) | 0%(0/21,162) | 36 months† | Multivessel CAD | 12 months of DAPT (aspirin and ticagrelor) | 36 months of DAPT (aspirin and ticagrelor) | Long-term DAPT reduced ischaemic events, but increased TIMI major bleeding (not ICH or fatal bleeding) in multivessel PCI patients |
| Patient-level meta-analysis of 8 RCTs13 -2019 | 20.8%(3,118/14,963) | 55.5%(8,312/14,963) | 24 months | ≥1 of the following: | 3 to 6 months of DAPT (aspirin and clopidogrel, prasugrel or ticagrelor) | 12 to 24 months of DAPT (aspirin and clopidogrel, prasugrel or ticagrelor) | Long-term DAPT in non-HBR patients reduced ischaemic events in both complex and non-complex PCI groups, but not among HBR patients, regardless of PCI complexity |
| 3 vessels treated, | |||||||
≥3 stents implanted, |
|||||||
≥3 lesions treated, |
|||||||
bifurcation with 2 stents implanted, |
|||||||
total stent length >60 mm, |
|||||||
| CTO PCI | |||||||
| IDEAL-LM trial87-2022 | 100%(818/818) | 40.4%(331/818) | 24 months | LM PCI | 4 months of DAPT (aspirin and clopidogrel, prasugrel or ticagrelor) | 12 months of DAPT (aspirin and clopidogrel, prasugrel or ticagrelor) | 4 months of DAPT was non-inferior to 12 months of DAPT with respect to the ischaemic composite endpoint in LM PCI patients |
| *Median follow-up time: 392 days. †Median follow-up time: 33 months. ACS: acute coronary syndrome; CAD: coronary artery disease; CTO: chronic total occlusion; DAPT: dual antiplatelet therapy; HBR: high bleeding risk; ICH: intracranial haemorrhage; LM: left main; MACE: major adverse cardiovascular events; PCI: percutaneous coronary intervention; RCT: randomised clinical trial; TIMI: Thrombolysis in Myocardial Infarction | |||||||
2. P2Y12 inhibitor monotherapy
P2Y12 inhibitor monotherapy from PCI to 1 year
Different types of P2Y12 inhibitors and varying timings of aspirin discontinuation (e.g., 1-3 months after PCI) have been tested in randomised trials, showing a satisfactory safety and efficacy profile overall (Table 2)48.
Clopidogrel monotherapy was investigated in three trials495051. Across these studies, clopidogrel monotherapy reduced major bleeding rates without increasing ischaemic events as compared with standard 12-month DAPT, regardless of PCI complexity525354. However, in a pooled analysis of nearly 6,000 patients, clopidogrel monotherapy after 1-month DAPT, compared with 12 months of DAPT, was associated with a numerical increase in cardiovascular events among patients with ACS, but not in patients with CCS, suggesting a possible safety issue with clopidogrel monotherapy in patients with ACS55.
In the “Comparative effectiveness of 1 month of ticagrelor plus aspirin followed by ticagrelor monotherapy versus a current-day intensive dual antiplatelet therapy in all-comers patients undergoing percutaneous coronary intervention with bivalirudin and BioMatrix family drug-eluting stent use” (GLOBAL LEADERS) trial, 23-month ticagrelor monotherapy after 1-month DAPT was compared with 12-month DAPT with ticagrelor or clopidogrel followed by aspirin monotherapy among 15,968 patients undergoing PCI56. In a post hoc analysis of the study, ticagrelor monotherapy significantly reduced the risk of the primary endpoint, a composite of all-cause death or new Q-wave MI, in patients with complex PCI but not in those with non-complex PCI features (p for interaction=0.015), a benefit that was mainly driven by a significant interaction for treatment effect in patients presenting with ACS; moreover, no significant difference in bleeding was shown57. Ticagrelor monotherapy after 3-month DAPT was evaluated in 7,119 high-risk patients enrolled in the Ticagrelor With Aspirin or Alone in High-Risk Patients After Coronary Intervention (TWILIGHT) trial and in 3,056 patients presenting with ACS in the Ticagrelor Monotherapy After 3 Months in the Patients Treated With New Generation Sirolimus Stent for Acute Coronary Syndrome (TICO) trial5859. In the TWILIGHT-COMPLEX substudy, a total of 2,342 patients underwent complex PCI and, in line with the parental trial, ticagrelor monotherapy was associated with a significant reduction of major bleeding and similar ischaemic risk compared with ticagrelor plus aspirin, irrespective of periprocedural complexity60. In the TICO trial, when the clinical characteristics (e.g., diabetes and chronic kidney disease) were combined with procedural complexity criteria, no significant interaction was evident for ticagrelor monotherapy after 3-month DAPT compared with 12-month DAPT with ticagrelor61. More recently, in a pooled analysis including 7,529 patients with ACS from the TICO and TWILIGHT trials, ticagrelor monotherapy significantly reduced major bleeding, without increasing adverse events, with no evidence of interaction according to PCI complexity62.
Prasugrel monotherapy was investigated in the recent Short and Optimal Duration of Dual Antiplatelet Therapy After Everolimus-Eluting Cobalt-Chromium Stent-3 (STOPDAPT-3) trial. In 1,230 patients with ACS or HBR undergoing complex PCI, low-dose prasugrel (3.75 mg/day) monotherapy showed similar ischaemic and bleeding outcomes at 1 month compared with low-dose prasugrel-based DAPT63. However, it should be noted that there was an excess of unplanned revascularisation and subacute definite or probable stent thrombosis in the aspirin-free group6364.
Two recent meta-analyses showed a consistent reduction in the risk of major bleeding during P2Y12 inhibitor monotherapy in patients undergoing complex PCI, without an increased risk of ischaemic events6566. Despite the accumulated evidence, the current guidelines recommend P2Y12 inhibitor monotherapy only in patients with ACS at high bleeding risk (Class IIb, Level of Evidence B) or in patients with CCS at high ischaemic risk in the absence of HBR features (Class IIb, Level of Evidence C) (Central illustration)1415.
Table 2. Studies comparing P2Y12 inhibitor monotherapy versus DAPT in patients undergoing complex PCI.
| Study - year | Complex PCI patients | ACS patients | Follow-up | Complex PCI criteria | P2Y12 inhibitor monotherapy regimen | DAPT regimen | Results |
|---|---|---|---|---|---|---|---|
| GLOBAL LEADERStrial57 -2019 | 28.6%(4,570/15,968) | 45.4%(7,260/15,968) | 24 months | ≥1 of the following: | 23-month ticagrelor monotherapy following 1-month DAPT (aspirin and ticagrelor) | 12-month aspirin monotherapy following 12-month DAPT (aspirin and ticagrelor or clopidogrel) | Ticagrelor monotherapy significantly reduced the risk of the primary ischaemic endpoint in complex PCI patients, but not in the non-complex PCI group |
| 3 vessels treated, | |||||||
| ≥3 stents implanted, | |||||||
| ≥3 lesions treated, | |||||||
| bifurcation with 2 stents implanted, | |||||||
| total stent length >60 mm | |||||||
| TWILIGHTtrial60 -2020 | 32.8%(2,342/7,119) | 64.8%(4,614/7,119) | 15 months | ≥1 of the following: | 12-month ticagrelor monotherapy following 3-month DAPT (aspirin and ticagrelor) | 12-month DAPT (aspirin and ticagrelor) | Ticagrelor monotherapy significantly reduced the risk of the primary bleeding endpoint in complex PCI patients without an increased risk of ischaemic events |
| 3 vessels treated, | |||||||
| ≥3 lesions treated, | |||||||
| bifurcation with 2 stents implanted, | |||||||
| total stent length >60 mm, | |||||||
| CTO PCI, | |||||||
| atherectomy device use, | |||||||
| LM PCI, | |||||||
| surgical bypass graft PCI | |||||||
| TICOtrial61 -2021 | 13.3%(409/3,056)* | 100%(3,056/3,056) | 12 months | ≥1 of the following†: | 9-month ticagrelor monotherapy following 3-month DAPT (aspirin and ticagrelor) | 12-month DAPT (aspirin and ticagrelor) | Ticagrelor monotherapy after short-duration DAPT was not associated with an increased risk of ischaemic or bleeding events in high-ischaemic risk patients |
| ≥3 stents implanted, | |||||||
| total stent length >60 mm, | |||||||
| bifurcation with 2 stents implanted, | |||||||
| LM PCI, | |||||||
| CTO PCI, | |||||||
| history of DM or CKD | |||||||
| SMART-CHOICEtrial52 -2021 | 16.6%(498/2,993) | 100%(2,993/2,993) | 12 months | ≥1 of the following: | 9-month P2Y12 inhibitor monotherapy (mostly clopidogrel) following 3-month DAPT (aspirin and mostly clopidogrel) | 12-month DAPT (aspirin and mostly clopidogrel) | P2Y12 inhibitor monotherapy, mostly clopidogrel, after short-duration DAPT was not associated with an increased risk of ischaemic events in complex PCI patients |
| 3 vessels treated, | |||||||
| ≥3 stents implanted, | |||||||
| ≥3 lesions treated, | |||||||
| bifurcation with 2 stents implanted, | |||||||
| total stent length >60 mm | |||||||
| Pooled population of STOPDAPT-253 -2021andSTOPDAPT-2 ACS trials54 -2023 | 16.7%‡(999/5,997) | 68.9%§(4,136/5,997) | 12 months | ≥1 of the following: | 11-month clopidogrel monotherapy following 1-month DAPT (aspirin and clopidogrel) | 12-month DAPT (aspirin and clopidogrel) | Clopidogrel monotherapy after short-duration DAPT showed comparable ischaemic and bleeding outcomes compared to 12-month DAPT in complex PCI patients |
| 3 vessels treated, | |||||||
| ≥3 stents implanted, | |||||||
| ≥3 lesions treated, | |||||||
| bifurcation with 2 stents implanted, | |||||||
| total stent length >60 mm, | |||||||
| CTO PCI | |||||||
| STOPDAPT-3 trial63 -2024 | 20.6%(1,228/5,966) | 75%(4,474/5,966) | 12 months | ≥1 of the following: | Low-dose prasugrel monotherapy | 1-month DAPT (aspirin and low-dose prasugrel) | Low-dose prasugrel monotherapy showed comparable ischaemic and bleeding outcomes compared to 1-month DAPT in the complex PCI cohort. However, an excess of unplanned revascularisation and subacute definite or probable stent thrombosis was found in the no-aspirin group |
| 3 vessels treated, | |||||||
| ≥3 stents implanted, | |||||||
| ≥3 lesions treated, | |||||||
| bifurcation with 2 stents implanted, | |||||||
| total stent length >60 mm, | |||||||
| CTO PCI | |||||||
| *Complex PCI patients: 409; non-complex PCI patients: 2,647. †Information for the post hoc analysis of the TICO trial refers to patients defined as at high ischaemic risk, which included those undergoing complex PCI, having chronic kidney disease, or diabetes mellitus. ‡Complex PCI patients from the STOPDAPT-2 cohort: 509 (16.9%); complex PCI patients from STOPDAPT-2 ACS: 490 (16.3%). §ACS patients from the STOPDAPT-2 cohort: 1,148 (38.1%); ACS patients from STOPDAPT-2 ACS: 2,988 (100%). ACS: acute coronary syndrome; CKD: chronic kidney disease; CTO: chronic total occlusion; DAPT: dual antiplatelet therapy; DM: diabetes mellitus; HBR: high bleeding risk; LM: left main; PCI: percutaneous coronary intervention | |||||||
P2Y12 inhibitor monotherapy after 1 year
Although aspirin remains the treatment of choice for long-term secondary prevention after PCI, P2Y12 inhibitor monotherapy is emerging as a novel strategy (Table 2, Table 3). A recent patient-level meta-analysis of 7 randomised trials compared P2Y12 inhibitor monotherapy versus aspirin monotherapy for long-term secondary prevention in patients with established coronary artery disease. P2Y12 inhibitor monotherapy was superior to aspirin monotherapy in terms of ischaemic outcomes, whereas the rate of major bleeding was similar between the two strategies67. In the Harmonizing Optimal Strategy for Treatment of Coronary Artery Stenosis-EXtended Antiplatelet Monotherapy (HOST-EXAM) trial, clopidogrel monotherapy was associated with lower risks of both thrombotic and bleeding events compared with aspirin among more than 5,000 patients. A subgroup analysis of PCI complexity showed consistent benefit of clopidogrel monotherapy with no significant interaction68. A landmark analysis of the GLOBAL LEADERS trial at 12-month follow-up showed that ticagrelor versus aspirin monotherapy significantly reduced ischaemic events (all-cause death, any MI, or any stroke), although it numerically increased the risk of bleeding. This result was consistent in patients undergoing both complex and non-complex PCI69. Of note, clopidogrel monotherapy has been endorsed by ESC guidelines on CCS as a safe and effective alternative to aspirin for long-term post-PCI secondary prevention (Class I, Level of Evidence A)15.
Table 3. Studies comparing other antithrombotic strategies versus a standard strategy in patients undergoing complex PCI.
| Study - year | Complex PCI patients | ACS patients | Follow-up | Complex PCI criteria | Antithrombotic experimental regimen | Standard regimen | Results |
|---|---|---|---|---|---|---|---|
| CHAMPION PHOENIXtrial45 -2018 | 83.3%(9,037/10,854) | 41.6%(4,518/10,854) | 48 hours | ≥1 of the following: | Cangrelor (30 µg/kg i.v. bolus followed by a 4 µg/kg/min infusion i.v.) | Clopidogrel (loading dose of 600 mg or 300 mg) | Cangrelor reduced MACE within 48 hours after PCIin both CCS and ACS, compared to an LD of clopidogrel in ADP receptor inhibitor-naïve patients |
| long lesions, | |||||||
| LM lesions, | |||||||
| bifurcation lesions, | |||||||
| thrombotic lesions, | |||||||
| calcified lesions, | |||||||
| multilesion PCI | |||||||
| COMPASStrial84 -2020 | 38.0%(3,775/9,862) | 0%(0/9,862) | 24 months | Multivessel PCI (patientswith previous PCI) | DPI with rivaroxaban (2.5 mg twice a day) and aspirin | Aspirin monotherapy | DPI reduced MACE and mortality compared with aspirin, but increased major bleeding in CCS patients with multivessel PCI |
| HOST REDUCE POLYTECHtrial76 -2022 | 31.0%(705/2,271) | 100%(2,271/2,271) | 12 months | ≥1 of the following: | DAPT with prasugrel dose de-escalation (5 mg once a day) following 1-month DAPT with the conventional prasugrel dose | 12-month DAPT with the conventional prasugrel dose(10 mg once a day) | Prasugrel dose de-escalation DAPT did not increase the risk of MACE, but decreased bleeding events compared with the conventional dose |
| ≥3 stents implanted, | |||||||
| ≥3 lesions treated, | |||||||
| bifurcation PCI | |||||||
| total stent length ≥60 mm, | |||||||
| LM PCI | |||||||
| heavily calcified lesions | |||||||
| HOST-EXAMtrial68 -2022 | 3%(152/4,717) | 0%(0/4,717) | 72 months* | 1 of the following: | Clopidogrel monotherapy following 12±6 months of DAPT with clopidogrel | Aspirin monotherapy following 12±6 months of DAPT with clopidogrel | Clopidogrel monotherapy showed a lower risk of both thrombotic and bleeding events compared with aspirin monotherapy |
| 3 vessels treated, | |||||||
| ≥3 stents implanted, | |||||||
| ≥3 lesions treated, | |||||||
| bifurcation with 2 stents implanted, | |||||||
| total stent length >60 mm, | |||||||
| CTO PCI | |||||||
| TALOS-AMItrial77 -2024 | 29.2%(788/2,697)†‡ | 100%(2,697/2,697) | 12 months | ≥1 of the following: | 11-month clopidogrel-based DAPT (aspirin and clopidogrel) following 1 month of ticagrelor-based DAPT (aspirin and ticagrelor) | 12-month ticagrelor-based DAPT (aspirin and ticagrelor) | An unguided de-escalation strategy to clopidogrel-based DAPT reduced bleeding events withouta significant difference in ischaemic outcomes, regardless of the presence of high ischaemic risk features |
| history of DM or CKD, | |||||||
| multivessel PCI, | |||||||
| ≥3 lesions treated, | |||||||
| total stent length >60 mm, | |||||||
| ≥3 stents implanted, | |||||||
| LM PCI, | |||||||
| bifurcation with 2 stents implanted | |||||||
| ALPHEUStrial42 -2024 | 48.3%(910/1,866) | 0%(0/1,866) | 1 month | ≥1 of the following: | Ticagrelor-based DAPT (aspirin and ticagrelor) | Clopidogrel-based DAPT (aspirin and clopidogrel) | Ticagrelor-based DAPT did not reduce periprocedural MI and ischaemic events compared with clopidogrel in CCS patients undergoing complex PCI |
| total stent length >60 mm, | |||||||
| bifurcation with 2 stents implanted, | |||||||
| LM PCI, | |||||||
| bypass graft PCI, | |||||||
| CTO PCI, | |||||||
| use of atherectomy, | |||||||
| multiple stents implanted | |||||||
| *Median follow-up time: 5.8 years. †Complex PCI patients: 788; non-complex PCI patients: 1,909. ‡Information for the post hoc analysis of the TALOS-AMI trial refers to patients defined as at high ischaemic risk, which included those undergoing complex PCI, and having a history of diabetes mellitus or chronic kidney disease. ACS: acute coronary syndrome; ADP: adenosine diphosphate; CCS: chronic coronary syndrome; CKD: chronic kidney disease; CTO: chronic total occlusion; DAPT: dual antiplatelet therapy; DM: diabetes mellitus; DPI: dual pathway inhibition; LD: loading dose; LM: left main; MACE: major adverse cardiovascular events; MI: myocardial infarction; PCI: percutaneous coronary intervention | |||||||
3. Switching, escalation and de-escalation of antithrombotic therapies
Switching between antithrombotic drugs can involve both anticoagulant and antiplatelet agents. As a general approach, switching between anticoagulants should be avoided unless fondaparinux has been used before PCI14.
Switching between P2Y12 inhibitors can involve switching between two oral P2Y12 inhibitors (escalation, de-escalation, or change) or can involve an intravenous P2Y12 inhibitor (bridge or transition). Both escalation and de-escalation approaches can be guided or unguided, according to whether genetic tests to identify carriers of CYP2C19 loss-of-function alleles or platelet function tests are performed or not, respectively. In general, switching between oral P2Y12 inhibitors should occur with the administration of a loading dose in the setting of ACS, whereas reloading is not required in the context of CCS, with the exception of ticagrelor70. Despite the lack of data on complex PCI, a meta-analysis of both randomised and non-randomised trials showed that guided escalation is associated with a decreased risk of ischaemic events without a trade-off in bleeding71. In this respect, an expert consensus statement supported the use of platelet-function testing to escalate P2Y12 inhibitors in patients undergoing complex PCI (multivessel PCI, ≥3 stents implanted, bifurcation PCI with 2 stents, total stent length >60 mm, CTO PCI)72.
A de-escalation strategy has been tested exclusively in patients with ACS across 6 trials (2 functional-guided, 1 genotype-guided, 3 unguided trials)73. Except for one trial74, all the studies met their primary endpoint and showed significant reductions of net adverse clinical events and major bleeding, without increasing ischaemic events. Recently, four of these trials were pooled in an individual patient-data meta-analysis of 10,133 patients75. Guided or unguided de-escalation provided consistent results and was associated with both decreased ischaemic and bleeding endpoints. There was no interaction for either ischaemic or bleeding outcomes in patients undergoing multivessel versus single-vessel PCI nor in those undergoing PCI with ≥3 versus <3 implanted stents, which are considered proxies for complex PCI. Consistently, there was no interaction according to PCI complexity for both ischaemic and bleeding outcomes in the Harmonizing Optimal Strategy for Treatment of Coronary Artery Diseases Trial - Comparison of REDUCTION of prasugrEl Dose & POLYmer TECHnology in ACS Patients (HOST REDUCE POLYTECH) and TicAgrelor Versus CLOpidogrel in Stabilized Patients With Acute Myocardial Infarction (TALOS-AMI) trials, which showed reduced bleeding events with unguided prasugrel or ticagrelor de-escalation, respectively (Table 3)7677. Current ESC guidelines on ACS recommend a de-escalation strategy after 30 days to prevent bleeding (Class IIb, Level of Evidence A)14.
A transition from cangrelor to clopidogrel or prasugrel should occur at the end of cangrelor infusion, or 30 minutes before the cangrelor infusion is stopped when prasugrel is used. Transition from cangrelor to ticagrelor is not affected by drug-drug interaction and therefore can occur at any time during PCI707879.
4. Dual pathway inhibition
Dual pathway inhibition (DPI) consists of a simultaneous inhibition of both platelet activity and coagulation cascade by the administration of an antiplatelet agent combined with an anticoagulant drug. This strategy is aimed to target the residual cardiovascular ischaemic risk in patients who present with ACS and/or undergo coronary revascularisation80. The Apixaban for Prevention of Acute Ischemic and Safety Events (APPRAISE) trial showed that among patients with ACS with high-risk clinical features (44% treated with PCI), the addition of a full dose of apixaban (5 mg twice daily) to DAPT increased the risk of major bleeding without a significant reduction in ischaemic events as compared with standard DAPT81. The Anti-Xa Therapy to Lower Cardiovascular Events in Addition to Standard Therapy in Subjects with Acute Coronary Syndrome 2–Thrombolysis in Myocardial Infarction 51 (ATLAS ACS 2–TIMI 51) trial tested rivaroxaban on a background of single or dual antiplatelet therapy in patients with recent ACS and showed a significant reduction of ischaemic events and mortality with low-dose rivaroxaban (2.5 mg twice daily) as compared to placebo, counterbalanced by an increase of major bleeding82. Similarly, in the Cardiovascular OutcoMes for People Using Anticoagulation StrategieS (COMPASS) trial, a low dose of rivaroxaban (2.5 mg twice daily) on top of aspirin decreased the rates of major adverse cardiovascular events and cardiovascular mortality, at the expense of bleeding events, in patients with CCS (60% with a clinical history of MI or peripheral artery disease)83. This benefit was preserved in a prespecified subgroup analysis including patients with CCS treated with PCI, of whom a substantial proportion (38%) underwent complex, multilesion PCI84. Based on the available evidence (Table 3), a DPI strategy with rivaroxaban in addition to aspirin should be considered for extended long-term (beyond 12 months) prevention in patients without HBR and with high ischaemic risk, including those presenting with complex PCI (Class IIa, Level of Evidence A) according to current ESC guidelines on the management of ACS (Central illustration)14.
Specific settings
Some complexity features, such as LM, bifurcation and CTO lesions, have been associated with worse prognostic impact and increased rates of ischaemic events. For this reason, some studies have evaluated the optimal antithrombotic therapy in these subsets of patients5. On the other hand, patients with a chronic indication to oral anticoagulation undergoing complex PCI represent a unique cohort with a higher bleeding risk.
1. Left main disease
LM PCI represents one of the most challenging scenarios in view of the large area of myocardium at risk with a potentially catastrophic impact of thrombotic complications related to LM stenting. Indeed, unprotected LM disease can substantially affect myocardial supply and has a critical role in long-term outcomes in patients undergoing PCI, with a higher long-term risk of ischaemic events85. In a post hoc analysis of the Evaluation of XIENCE Versus Coronary Artery Bypass Surgery for Effectiveness of Left Main Revascularization (EXCEL) trial, continuation of DAPT beyond 1 year was not associated with improved outcomes, and trends toward harm were noted with extended DAPT after propensity adjustment86. In the randomised Improved Drug-Eluting stent for All-comers Left Main (IDEAL-LM) study, among 818 patients undergoing PCI of the LM, a biodegradable-polymer everolimus-eluting stent followed by 4 months of DAPT resulted in non-inferior outcomes compared to a durable-polymer everolimus-eluting stent followed by 12 months of DAPT. However, major adverse cardiovascular events and its individual components trended numerically higher in the biodegradable-polymer stent with the 4-month DAPT group87. Along the same line, in a prospective observational study enrolling 3,865 patients undergoing LM PCI and at low bleeding risk, a longer duration (>12 months) of DAPT was associated with a significant reduction of ischaemic events without a concomitant increase in clinically relevant bleeding as compared with ≤12-month DAPT88. Interestingly, in a large retrospective analysis including 700 patients undergoing LM PCI, the rate of target lesion failure in patients treated with a two-stent technique was significantly higher than in the one-stent group only when DAPT was interrupted before 1 year89. Given the available evidence (Table 1), even if LM PCI does not appear to be a treatment modifier for oral antithrombotic therapy, it is well recognised as a procedural risk enhancer for ischaemic events. For this reason, LM PCI is considered an ischaemic risk condition, allowing for extended antithrombotic therapy (with DAPT or DPI) beyond 6 and 12 months in patients with CCS and ACS, respectively (Class IIa, Level of Evidence A)1415. In patients with CCS undergoing complex LM PCI, potent P2Y12 inhibitors may be an alternative to clopidogrel in selected cases (Class IIb, Level of Evidence C)15.
2. Bifurcation PCI
Bifurcation lesions have a critical prognostic impact on ischaemic outcomes with a 2-year major adverse cardiovascular events rate of 10% and a higher risk of stent thrombosis4585. Bifurcation stenting is considered an ischaemic risk criterion, justifying the recommendation of extended antithrombotic therapy (with both DAPT or DPI) beyond 6 and 12 months in patients with CCS and ACS, respectively (Class IIa, Level of Evidence A), and the opportunity to replace clopidogrel with potent P2Y12 inhibitors in patients with CCS (Class IIb, Level of Evidence C)1415. In 2,082 patients who underwent bifurcation stenting in the Coronary Bifurcation Stenting (COBIS) II registry and who were event-free at 12 months, prolonged DAPT was associated with a reduced incidence of all-cause death, MI and stent thrombosis at 4-year follow-up compared with a short-term strategy (<12 months). Interestingly, the beneficial role of prolonged DAPT was not significantly affected by lesion location or stenting technique90. Similarly, in the European Bifurcation Club registry, prolonged DAPT was associated with a significantly lower risk of ischaemic events as compared with both the short-term DAPT group (<6 months) and standard DAPT group (6-12 months) at 2-year follow-up. Furthermore, event-free survival was significantly lower in the group of DAPT duration <6 months91. However, the currently available evidence about antithrombotic therapy in bifurcation PCI is not derived from major dedicated trials, and observational studies may be inexorably biased. A decision-making algorithm for DAPT duration based primarily on the clinical presentation, the baseline bleeding risk, the stenting strategy, and the possible use of intracoronary imaging in patients who are not candidates for anticoagulant therapy has recently been proposed and may inform clinician decisions92.
3. CTO PCI
As previously described, patients with a CTO are considered at increased ischaemic risk. Notably, extensive calcification, a common CTO lesion feature, hinders stent expansion and leads to a higher risk of both acute and late stent thrombosis. Most of the time, CTO PCI is performed as a scheduled intervention in patients with CCS; thus, aspirin and clopidogrel are the standard DAPT regimen, even if ticagrelor or prasugrel may be considered (Class IIb, Level of Evidence C). Regarding the DAPT duration, prolonged DAPT (>6 months) should be considered according to European guidelines (Class IIa, Level of Evidence A)1415. Lee et al compared ≤12-month with >12-month DAPT in 512 patients undergoing CTO PCI. In a propensity score-matched population, the rate of ischaemic events was similar between the two groups93. More recently, Sachdeva et al found that prolonged DAPT (>12 months) was associated with a lower incidence of death or MI without an increase in bleeding rate in 1,069 patients undergoing CTO PCI94. Further dedicated prospective studies are required to assess the optimal antithrombotic strategy in this distinct subgroup.
4. SVG PCI
PCI of SVG disease represents a technical challenge considering that the pathophysiological mechanisms are distinct from those of native coronary artery disease. Despite their poor mid- and long-term patency rates, venous grafts are still commonly used in patients undergoing surgical myocardial revascularisation95. SVG PCI is frequently performed in the setting of ACS. Among 8,582 patients enrolled in the ADAPT-DES study, 405 (4.7%) underwent SVG PCI. In this cohort, patients who underwent SVG PCI had a higher risk of ischaemic events compared with patients undergoing PCI of native coronary arteries. For this reason, SVG PCI is considered, in some cases, a complex PCI criterion; prolonged antithrombotic therapy therefore provides potential benefits96. However, further studies are warranted to investigate this particular cohort, and, to date, no specific recommendations are available.
5. Patients with atrial fibrillation requiring oral anticoagulation
Patients with atrial fibrillation (AF) requiring oral anticoagulants (OACs) undergoing PCI are at increased risk for thromboembolic and bleeding events. Current guidelines recommend triple antithrombotic therapy (TAT) consisting of DAPT with clopidogrel plus an OAC agent for a short peri-interventional 1-week time frame, followed by dual antithrombotic therapy (DAT) with clopidogrel plus OAC for up to 6 or 12 months after PCI for patients with CCS and ACS, respectively (Class I, Level of Evidence A)141597. This recommendation largely relies on four major trials comparing DAT, mainly based on non-vitamin K antagonist oral anticoagulants (NOACs), with vitamin K antagonist (VKA)-based TAT in patients with AF presenting with ACS and/or undergoing PCI9899100. Across these trials, DAT was associated with a significant reduction of major bleeding, a similar incidence of ischaemic stroke, numerically higher risks of MI and stent thrombosis, and an overall neutral effect on all-cause mortality. A post hoc analysis of Open-Label, Randomized, Controlled, Multicenter Study Exploring Two Treatment Strategies of Rivaroxaban and a Dose-Adjusted Oral Vitamin K Antagonist Treatment Strategy in Subjects with Atrial Fibrillation who Undergo Percutaneous Coronary Intervention (PIONEER AF-PCI) and Randomized Evaluation of Dual Antithrombotic Therapy with Dabigatran versus Triple Therapy With Warfarin in Patients With Nonvalvular Atrial Fibrillation Undergoing Percutaneous Coronary Intervention (RE-DUAL PCI) showed consistent risk estimates between a DAT and/or NOAC-based strategy as compared with VKA-based TAT regardless of PCI complexity101102. However, the individual trials were underpowered for ischaemic outcomes, and two large meta-analyses found a significant association between DAT and the risk of stent thrombosis occurrence103104. For this reason, when the ischaemic risk outweighs the bleeding risk, TAT can be extended up to 1 month after PCI in patients with CCS (Class IIa, Level of Evidence B) and ACS (Class IIa, Level of Evidence C). ESC guidelines on both ACS and CCS provide several high ischaemic risk criteria for extended treatment with TAT that include complex PCI characteristics: diffuse multivessel disease, implantation of ≥3 stents, treatment of ≥3 lesions, total stent length >60 mm, bifurcation with two stents implanted, and treatment of a CTO1415. Furthermore, European guidelines on ACS indicate that continuation with one antiplatelet agent (aspirin or clopidogrel) beyond 1 year after ACS may be considered for patients having high-risk features of stent-driven recurrent ischaemic events. A similar approach is endorsed by the North American consensus recommending long-term continuation of single antiplatelet therapy only for selected patients at high risk for ischaemic recurrence and low bleeding risk14105. These recommendations should be interpreted, however, in view of recent trials showing a reduced risk of major bleeding with sole continuation of the OAC agent in the longstanding phase of coronary artery disease106.
Future perspectives
As the landscape of antithrombotic therapy continues to evolve, several innovative agents and strategies are poised to enhance the outcomes of patients undergoing complex PCI. The development of subcutaneous, self-administered P2Y12 inhibitors like selatogrel, currently being tested in the SOS-AMI trial (ClinicalTrials.gov: NCT04957719), and the novel subcutaneously administered GPI zalunfiban (RUC-4), being evaluated in the CELEBRATE trial (NCT04825743), represents a shift towards more accessible, rapid-acting therapies for acute MI, which may revolutionise early intervention for patients with complex coronary artery disease.
Tailored antithrombotic strategies are also being explored specifically for high-risk complex PCI populations, such as switching from ticagrelor- to clopidogrel-based DAPT at 6 months (TAILORED-CHIP; ClinicalTrials.gov: NCT03465644), utilising low-dose prasugrel or low-dose ticagrelor DAPT (E5TION; NCT04734353), prolonging DAPT with aspirin and prasugrel (ATTEMPT; NCT04014803), and comparing long-term clopidogrel and aspirin monotherapy (SMART-CHOICE3; NCT04418479). Additionally, the advent of factor XIa inhibitors promises to target residual thrombotic risk while decoupling haemostasis and thrombosis, mirroring the benefits of DPI, but with a potentially lower risk of bleeding. Among the factor XIa inhibitors in development, milvexian is currently being tested in the phase III LIBREXIA-ACS trial (NCT05754957), enrolling patients with ACS undergoing PCI3. As these novel strategies continue to be validated through clinical trials, they have the potential to transform the standard of care for complex PCI, offering more personalised, safer, and more effective treatment options for high-risk patients. Furthermore, these studies could be helpful to better define the profile and unique definition of patients undergoing complex PCI.
Conclusions
As complex PCI is associated with a higher ischaemic risk, adjunctive antithrombotic therapy plays a key role and should be part of the optimal revascularisation strategy. Some very high-risk patients may derive clinical benefit from extending DAPT beyond the period mandated by clinical guidelines, but bleeding risk status should always be considered in the decision. New strategies including early aspirin withdrawal followed by monotherapy with a potent P2Y12 inhibitor or implementation of DPI have shown promising results and may be attractive strategies in this setting, particularly in patients at high bleeding or ischaemic risk, respectively. To summarise, in patients undergoing complex PCI, the evaluation of bleeding risk is pivotal: in patients with HBR features, ticagrelor or clopidogrel monotherapy, after a short course of DAPT, should be the antithrombotic strategy of choice; conversely, in patients without bleeding concerns, prolonged DAPT represents the gold standard antithrombotic therapy, even if P2Y12 inhibitor monotherapy or DPI are promising strategies in this setting. Hence, we have proposed an algorithm to guide antithrombotic therapy strategies integrating bleeding risk and indication to oral anticoagulation after complex PCI (Central illustration). However, a standardised definition of complex PCI is still lacking, which partly hampers the comparability of previous studies. Moreover, it is important to acknowledge that the available evidence on the safety and efficacy profiles of antithrombotic strategies in complex PCI, as well as in specific subsets, is derived from subgroup analyses of randomised trials, which might be underpowered to assess heterogeneity in the treatment effect between patients undergoing complex and non-complex PCI. Although the definition of complex PCI is increasingly becoming “prespecified” in trial protocols, the presence of subgroup phenomena can bias the interpretation of results. Finally, it is also important to recognise that some PCI criteria for complexity are unplanned or might not become evident upon PCI completion. Even if this aspect poses challenges, dedicated randomised trials for patients undergoing complex PCI are warranted to find the optimal antithrombotic strategy in this specific population and, in this way, optimise outcomes.
Acknowledgements
D.S. Castiello, A. Oliva, G. Andò, G. Niccoli, and F. Pelliccia conceived and designed the project. R. Piccolo, E. Moscarella, R.A. Montone, F. Gragnano, and I. Porto supervised the project. P. Calabrò, S. De Rosa, E. Fabris, and G. Sinagra contributed to the acquisition, analysis and interpretation of data. D.S. Castiello, A. Oliva, and R. Piccolo drafted the manuscript. C.A.M. Spaccarotella, G. Esposito, C. Indolfi, and P.P. Filardi critically revised the manuscript for important intellectual content and the final approval of the version to be published. All authors have read and agreed to the published version of the manuscript.
Funding
This work is supported by a research grant from the Italian Ministry of Education (PRIN PNRR P2022RJS7X and PRIN 2022497RZ4 to R. P.).
Conflict of interest statement
The authors have no conflicts of interest to declare that could have appeared to influence the work reported in this paper.

