
Abstract
Aims: We sought to investigate the relevance of myocardial fibrosis, assessed by mid-wall fibrosis risk (MFR) score, with respect to left ventricular (LV) reverse remodelling following transcatheter aortic valve replacement (TAVR).
Methods and results: Between January 2010 and March 2015, we enrolled 207 patients in whom baseline MFR, which includes age, sex, high-sensitivity cardiac troponin I, presence of strain pattern on electrocardiography, and peak aortic valve velocity, as well as one-year follow-up echocardiography was available. LV reverse remodelling was defined as a >10% reduction in LV end-diastolic volume index (LVEDVi). A higher MFR score (≥52) was associated with increased LVEDVi and with decreased LV ejection fraction as well as higher baseline NT-proBNP levels (p<0.05 for all). One year after the TAVR procedure, a higher MFR score was associated with a decreased probability of LV reverse remodelling (OR 0.33, 95% CI: 0.23-0.87; p=0.03), which was independent of baseline echocardiographic parameters and comorbidities. In contrast, there was no significant difference in five-year mortality between patients with lower and higher MFR scores (57.9% vs 60.5%, p=0.66).
Conclusions: A higher MFR score is associated with reduced LV reverse remodelling at one-year follow-up, whereas the MFR score does not appear to correlate with long-term mortality after TAVR.
Introduction
The increase in afterload imposed by aortic stenosis (AS) leads to adaptive left ventricular (LV) remodelling, resulting in LV hypertrophy and consecutively impaired systolic as well as diastolic LV function and poor prognosis1,2. The histopathological causes of LV remodelling are the loss of viable myocardium and increased fibrosis, which have been found to correlate with increased adverse events in patients with AS3,4,5. Additionally, myocardial fibrosis (MF) appears to be associated with a lack of improvement in systolic and diastolic LV function after surgical aortic valve replacement (SAVR), suggesting an impact of MF on LV reverse remodelling6,7. Recently, a clinical risk score has been introduced allowing the estimation of MF in patients with AS. The so-called mid-wall fibrosis risk (MFR) score has been found to predict adverse outcomes in moderate to severe asymptomatic AS8. In the context of transcatheter aortic valve replacement (TAVR), reduction of afterload has been shown to be linked to reverse remodelling, including a decrease of LV volume, a regression of LV mass, and an improvement of LV systolic function9,10. Despite its clinical relevance, little is known about the impact of myocardial fibrosis following TAVR, especially on LV reverse remodelling.
In this study, we therefore sought to evaluate the impact of the MFR score on a) LV reverse remodelling, b) improvement of LV systolic function, as well as c) the long-term outcome in patients undergoing TAVR. Furthermore, we aimed to establish a correlation of potential biomarkers of MF with the MFR score in a subgroup of patients.
Methods
We performed a retrospective observational cohort study of patients with severe, symptomatic AS who underwent TAVR from May 2009 to March 2015 at the Heart Center of the University Hospital, Bonn, Germany. To evaluate the impact of LV remodelling, only those patients who underwent follow-up echocardiography one year after TAVR were included. Patients with incomplete baseline data, and those who were not available for assessment of LV function, were excluded. Also, patients in whom the MFR score could not be adequately calculated, that is, patients who were missing a baseline electrocardiogram (ECG) or had a pacemaker ECG were excluded. Likewise, patients with post-TAVR pacemaker dependency at follow-up were excluded to avoid an underestimation or misjudgement of LV ejection fraction (LVEF). Clinical endpoints included all-cause mortality as well as complications (as defined by VARC-2), including minor and major vascular complications as well as paravalvular leakage11.
This study was conducted retrospectively from patients enrolled in a local TAVR registry, which was approved by the institutional ethics committee of the University of Bonn and was conducted in accordance with the Declaration of Helsinki. All patients provided written informed consent to a local TAVR registry.
MID-WALL FIBROSIS RISK SCORE
To assess the presence of MF, an MFR score was calculated using an app-based calculator (Calculate; QxMD Medical, Vancouver, BC, Canada)4. As previously published, the MFR score includes the following parameters: age, sex, high-sensitivity cardiac troponin I, the presence of strain pattern on the ECG, and peak aortic valve velocity. To evaluate the presence of an ECG strain pattern, a standard 12-lead ECG taken before TAVR was analysed. The presence of ECG strain was defined as a concave down-sloping ST depression (>1 mm) with asymmetrical T-wave inversion in the lateral leads12.
ECHOCARDIOGRAPHIC MEASUREMENT
Standard, two-dimensional echocardiographic assessments were performed at baseline as well as one year after the procedure, using the GE Vivid E9 system (GE Healthcare, Milwaukee, WI, USA) or Philips iE33 (Philips Healthcare, Best, the Netherlands). Chamber volume, systolic function, and wall thickness were assessed according to the current guidelines of the American Society of Echocardiography and the European Society of Echocardiography13,14. LV end-diastolic volume (LVEDV), LV end-systolic volume (LVESV), and LV mass (LVM) were divided by body surface area to calculate the respective indices (i.e., LVEDVi, LVESVi, LVMi). Based on earlier studies investigating LV reverse remodelling, we defined LV reverse remodelling as a reduction of the LVEDVi by >10%9,15.
BIOMARKERS OF FIBROSIS
We measured the levels of galectin-3, growth differentiation factor (GDF)-15, and soluble ST2 (sST2). Galectin-3 has been shown to play a role in the development of MF, by stimulating macrophage migration and fibroblast proliferation. The levels of galectin-3 were assessed using an enzyme-linked immunosorbent assay (BG Medicine, Foxboro, MA, USA)16. GDF-15, measured by using an ELISA assay (R&D Systems, Minneapolis, MN, USA), has been found to be associated with a lack of reverse remodelling after TAVR17. Soluble ST2, analysed by using a high-sensitivity Presage® ST2 assay (Life Biomedical, Cambridge, UK), is a member of the interleukin-1 receptor family and is thought to be associated with cell proliferation and inflammatory states18.
STATISTICAL ANALYSIS
The study population was divided into two groups according to a cut-off value for the MFR score by the receiver operating characteristic (ROC) curve for the reduction of LVEDVi by >10%. Continuous variables with a normal distribution are expressed as the mean±standard deviation. Non-normally distributed variables are presented as median values with an interquartile range (IQR). Categorical variables are shown as frequencies and percentages.
Logistic regression analysis was used to examine associations between the MFR score and LV reverse remodelling. Diabetes, hypertension, atrial fibrillation, coronary artery disease, N-terminal pro-B-type natriuretic peptide (NT-proBNP) level, New York Heart Association (NYHA) functional Class IV, LVEDVi, LVEF, LVMi, aortic valve area at baseline, paravalvular leakage ≥2+, and the MFR score were introduced into the final multivariate model; this was based on clinical knowledge of LV remodelling in AS patients7,9,15.
To estimate five-year mortality after TAVR, we performed a survival analysis using the Kaplan-Meier method. Based on previously published data demonstrating the prognostic relevance of the MFR score in patients with severe AS, we repeated the analysis with another cut-off of the MFR score (i.e., 57)8.
Two-tailed p-values <0.05 were considered to denote statistical significance. All statistical analyses were performed using EZR version 1.37 (Saitama Medical Center, Saitama, Japan), which is a graphical user interface for R (R Foundation for Statistical Computing, Vienna, Austria)19.
Results
STUDY POPULATION
For this study, data from 207 patients who underwent transfemoral TAVR were analysed. Overall, the mean age was 81±6 years and 51% of patients were male. The mean STS score and logistic EuroSCORE were 6.9±5.2% and 19.6±13.3%, respectively.
The median MFR score for the whole cohort was 53.3, IQR [26.8, 90.2]. With the use of ROC analysis, an MFR score of 52 was determined as the cut-off value for predicting LV reverse remodelling. Using this calculation, 106/207 patients (51.2%) displayed an MFR score ≥52. These patients were more often male, had more frequently undergone percutaneous coronary intervention, and had higher surgical risk scores than those with an MFR score <52 (Table 1). Regarding procedural characteristics, patients with a higher MFR score had a significantly larger implanted valve size (p<0.001). There were no significant differences between groups regarding the type of valve or in VARC-2 defined complications (Supplementary Table 1).
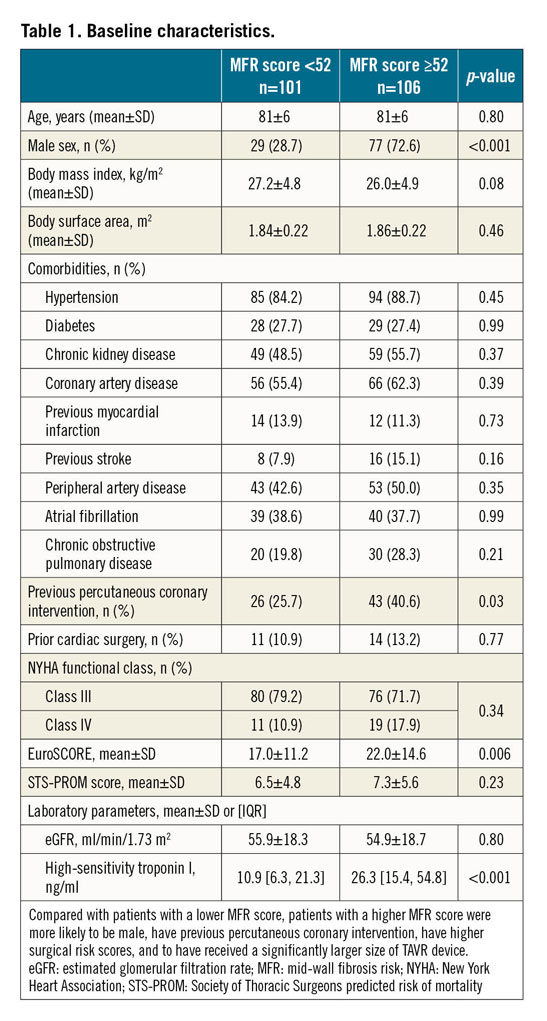
ASSOCIATION BETWEEN MFR SCORE AND ECHOCARDIOGRAPHIC PARAMETERS
Overall, the patient cohort presented with a normal range of LVEDVi (68.2±25.0 ml/m2), a preserved LVEF (53.0±13.4%), and an increased LVMi (131.3±44.5 g/m2) as well as a certain degree of diastolic dysfunction (E/A: 1.3±0.8; E/e’: 24.2±12.9). Of note, compared to patients with a lower MFR score, patients with a higher MFR score had a significantly greater LVEDVi (64.6±20.5 ml/m2 vs 71.6±28.4 ml/m2, p=0.04) and lower LVEF (55.0±11.8% vs 51.0±14.6% p=0.03), while there were no significant differences in LVMi or markers of diastolic dysfunction (i.e., E/A or E/e’) (Supplementary Table 2).
At one-year follow-up, a significant reduction in LVEDVi, an increase in LVEF, and a regression of LVMi were observed in the overall cohort (Supplementary Table 2). Among the cohort, 101/207 (48.8%) patients showed LV reverse remodelling, defined as a reduction in LVEDVi >10%. The relative change in LVEDVi (delta LVEDVi/baseline LVEDVi) after one year was significantly lower in patients with a higher MFR score (median –20.3%, IQR [−40.3, 4.3] vs median –14.4%, IQR [–36.1, 8.1], p<0.001) than those with a lower MFR score. Additionally, the MFR score was significantly lower in patients in whom LV reverse remodelling was observed, compared to those without remodelling (median 50.2, IQR [26.1, 79.0] vs median 54.7, IQR [29.5, 94.5], p<0.001) (Supplementary Table 3). After adjusting for baseline characteristics and echocardiographic parameters, a higher MFR score was associated with a decreased probability of LV reverse remodelling (OR 0.33, 95% CI: 0.23-0.87; p=0.03) (Table 2). The association remained significant in the sensitivity analysis with the MFR score as a continuous variable (Supplementary Table 4), which demonstrated that higher MFR scores were significantly associated with less reduction in LVEDVi (p=0.03) and regression in LVMi (p=0.004).
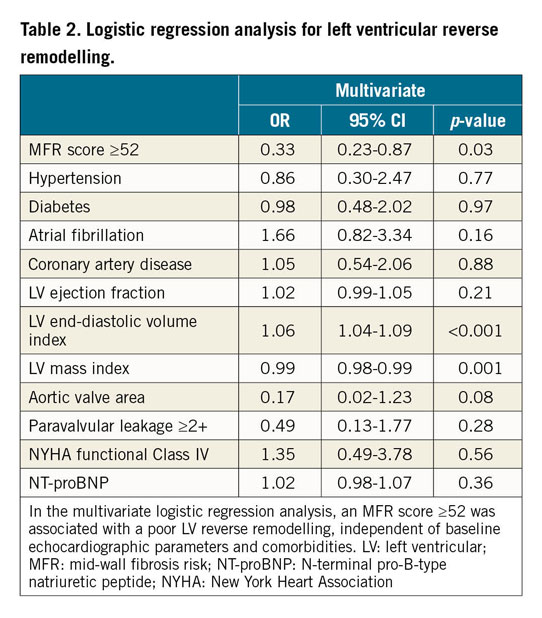
In contrast, no significant difference in the change in LVEF was seen in patients with lower or higher MFR scores (2.9±11.4% vs 5.7±12.6%, p=0.10). Also in this context, no significant correlation was seen between the MFR score and the change in LVEF (p=0.44).
LONG-TERM SURVIVAL BEYOND ONE YEAR AFTER TAVR
At one year after TAVR, clinical symptoms as per the NYHA classification were similar between patients with lower and higher MFR scores (NYHA Class III/IV: 13.8% vs 19.0%, p=0.43). The median duration of follow-up was 1,550 days, IQR1,2,101, with a yearly mortality rate (beyond one year after TAVR) of 9.3% per year. Regarding five-year mortality, there was no significant difference between the patients with lower and higher MFR scores (57.9% vs 60.5%, p=0.66) (Figure 1). Similarly, an additional analysis using another previously published prognostic cut-off of the MFR score (i.e., 57) revealed no significant differences in patients with lower or higher MFR scores (56.0% vs 63.1%, p=0.26).
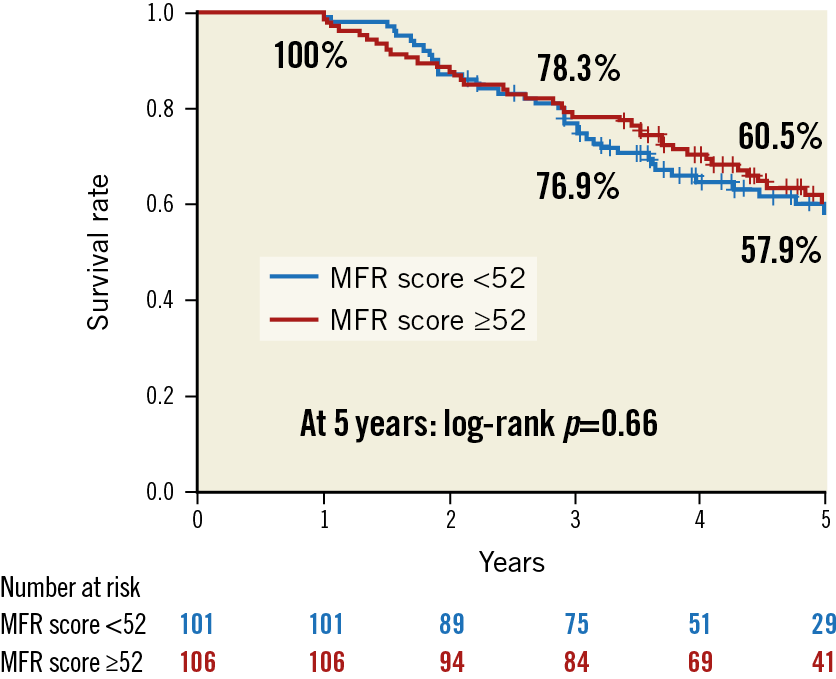
Figure 1. Kaplan-Meier analysis for five-year survival in patients with higher or lower MFR score. At five-year follow-up, there was no significant difference in mortality between patients with lower (<52) and higher (≥52) MFR scores (estimated survival rate: 57.9% vs 60.5%; p=0.66).
MFR AND CIRCULATING MARKERS OF FIBROSIS
Several potential biomarkers of MF were available for analysis in a subgroup of 116 patients (56% of the cohort). There were no significant differences between patients with higher and lower MFR scores with regard to circulating markers of fibrosis (Supplementary Table 5). Furthermore, no significant correlation between the MFR score and potential biomarkers of fibrosis could be observed (soluble ST2: r=–0.08; p=0.31; GDF-15: r=0.06; p=0.52; galectin-3: r=0.03; p=0.71). In contrast, the MFR score was positively associated with NT-proBNP (r=0.29; p<0.001).
Discussion
In AS, MF is the result of histopathological myocyte death and LV scarring, leading to LV remodelling and consequently LV dysfunction4,7. The recently published MFR score allows an estimation of MF in patients with aortic stenosis by integrating a number of clinical, echocardiographic and serological characteristics8. To the best of our knowledge, this study is the first report to evaluate the impact of MF (as determined by the MFR score) on LV reverse remodelling and on long-term outcome after TAVR.
MYOCARDIAL FIBROSIS AND LV REVERSE REMODELLING
The role of MF is well described in the context of both AS and SAVR. In fact, several studies have reported an association of MF with LV remodelling in patients with AS1,2. Furthermore, a negative impact of MF on LV reverse remodelling has been described in patients undergoing SAVR4,5,9. However, little is known about the impact of MF on LV reverse remodelling following TAVR. In line with a prior study evaluating MF by means of the MFR score in patients with AS8, in the present study there was a correlation of higher MFR scores with increased LVEDVi and LVMi at baseline, which indicates the applicability of the MFR score in patients undergoing TAVR. In addition, we found that a higher MFR score was associated with reduced LV reverse remodelling.
In contrast to prior studies on the impact of MF on the recovery of LV function after SAVR6, we did not observe a link between the MFR score and improvement of LVEF. A possible explanation could be related to paravalvular leakage after TAVR20, interfering with LV recovery and the possible association of the MFR score and improvement of LVEF. A sub-analysis of the NOTION trial has found a less pronounced degree of LV remodelling in patients undergoing TAVR with a self-expanding prosthesis as compared to SAVR. Whereas the authors attributed this to the presence of relevant paravalvular leakage and the need for permanent pacemakers21, in the present study an adjustment to include paravalvular leakage (≥2+) did not statistically influence our findings. In any case, the lack of association of the MFR with an improvement of LVEF highlights the difficulty of predicting the course of LVEF after TAVR22.
MYOCARDIAL FIBROSIS AND CIRCULATING BIOMARKERS
Of interest, in the present study, no correlation between the previously reported biomarkers (i.e., galectin-3, GDF-15, sST2) and the MFR score was observed. This observation stands in contrast to prior publications, which show a link between these biomarkers and MF. For example, sST2 has been shown to be a surrogate marker of LV remodelling as well as diastolic dysfunction in patients with AS18. Furthermore, Kim et al reported a correlation between levels of GDF-15 and the recovery of LV function, defined as an increase in global longitudinal strain, in patients undergoing TAVR17. Whereas a similar association was seen between the MFR score and LV reverse remodelling in our analysis, no direct correlation of the MFR score and these biomarkers could be established.
One possible explanation could be the extensive degree of MF present in our cohort. In fact, our cohort exhibited an MFR score which was at the high end of the intermediate score range (7 to 57) in the original publication by Chin et al8. It is therefore conceivable that the degree of MF and the already significantly elevated concentrations of the above-mentioned biomarkers in our cohort prevented the identification of a correlation.
Still, in line with previous studies on MF in the context of AS4,7, we found a positive correlation between the MFR score and NT-proBNP levels.
LONG-TERM SURVIVAL AFTER TAVR
Prior cohort studies have reported associations between MF and long-term adverse outcomes in patients with AS4,5. Although the original study by Chin et al demonstrated a predictive value of the MFR score on adverse outcomes in patients with AS4, in the present study five-year mortality after TAVR was not influenced by the MFR score. In consequence, it may be speculated that the treatment of AS in patients undergoing TAVR which leads to reverse LV remodelling and improved LVEF7,23 may have attenuated the impact of MF on clinical outcomes. Supporting this notion, a more recent cohort study of 31,199 patients undergoing TAVR reported that one-year mortality was not influenced by the type and extent of LV remodelling10. Alternatively, physiological and biological conditions (e.g., old age or frailty) in patients undergoing TAVR may have contributed to the long-term clinical outcomes observed. As a matter of fact, the long-term prognosis after TAVR appears to be evenly dependent on cardiovascular and non-cardiovascular determinants24.
In addition, the fact that the mortality rates in both the lower and higher MFR score groups compared well to the long-term results from the PARTNER 1 study25 could prompt the conclusion that patients with severe symptomatic AS benefit from TAVR regardless of the extent of MF.
Limitations
First, the present study was conducted retrospectively, based on a single-centre database and a relatively small cohort. Second, a major limitation of this study is the fact that MF was not detected by routine cardiovascular magnetic resonance (CMR) imaging but was estimated by the MFR score. Therefore, we cannot directly confirm that the MFR score was similarly associated with MF. Nevertheless, the MFR score in the present study demonstrated the same associations with increased LVEDVi, LVMi, and decreased LVEF at baseline, as observed in the previous CMR studies. We are therefore confident that the MFR score acted as a specific marker for MF in the patients included in this study. Finally, we did not distinguish the cause of mortality (cardiovascular or non-cardiovascular).
Conclusions
While a higher MFR score was associated with decreased LV reverse remodelling at one year after TAVR, the score was not associated with a change in LVEF. Additionally, the MFR score did not seem to impact on long-term mortality in patients in whom one-year follow-up echocardiography was available. Despite its impact on LV remodelling, our findings suggest that there is a limited impact of MF on the clinical outcome in patients undergoing TAVR.
|
Impact on daily practice Myocardial fibrosis, as assessed by the MFR score, is associated with decreased LV reverse remodelling at one year after TAVR. By contrast, the MFR score is not associated with a change in LVEF or with five-year mortality. Our findings may suggest that patients with AS could potentially benefit from TAVR regardless of the degree of their MFR score. |
Acknowledgements
We thank Dr Meghan Campbell (scientific coordinator for the Heart Center Bonn, Bonn, Germany) for proofreading the manuscript.
Conflict of interest statement
N. Werner, G. Nickenig and J.M. Sinning have received speaker honoraria and research grants from Medtronic, Boston Scientific, Edwards Lifesciences, and Abbott. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

