
Abstract
Aims: In this study we aimed to test the hypothesis that left ventricular (LV) afterload reduction in severe aortic valve stenosis (AS) by transcatheter aortic valve implantation (TAVI) acutely improves coronary haemodynamics.
Methods and results: This was a prospective, pathophysiologic study in 40 patients with severe AS undergoing TAVI. Endpoints were determined invasively immediately before and after TAVI without altering coronary stenotic lesions if present. Myocardial hyperaemia was induced by intravenous adenosine. The primary study endpoints were coronary flow reserve (thermodilution-derived CFR), and fractional flow reserve (FFR). The secondary study endpoint was coronary collateral flow index (CFI) as obtained during a one-minute coronary balloon occlusion. CFR was 1.9±0.9 before TAVI and 2.0±1.0 after TAVI (p=0.72). FFR was 0.90±0.08 before TAVI and 0.93±0.08 after TAVI (p=0.0021). The TAVI-induced increase in FFR was related to a significant decrease in hyperaemic mean aortic pressure from 71±16 mmHg before TAVI to 67±15 mmHg after TAVI (p=0.0099). Hyperaemic CFI increased from 0.127±0.083 before to 0.146±0.090 after TAVI (p=0.0508).
Conclusions: CFR appears not to be acutely affected by LV afterload reduction among patients with severe AS in response to TAVI. However, it acutely improves FFR; this occurs via lowering of mean aortic pressure. Hyperaemic coronary collateral flow index tends to augment in response to TAVI.
Abbreviations
AS: aortic valve stenosis
CAD: coronary artery disease
CFI: collateral flow index
CFR: coronary flow reserve
CVP: central venous pressure
FFR: fractional flow reserve
IMRtrue: true index of microcirculatory resistance
LV: left ventricle
Pao: mean aortic pressure
PCI: percutaneous coronary intervention
Pd: mean distal coronary pressure
Poccl: mean coronary occlusive pressure
TAVI: transcatheter aortic valve implantation
Tmn: mean transit circulation time
Introduction
Left ventricular (LV) afterload is defined as systolic LV wall stress, which is the product of LV systolic cavity pressure and diameter relative to LV wall thickness1. In the course of gradually narrowing aortic valve stenosis (AS), the increasing LV pressure, and thus wall stress, is compensated for by augmented myocardial wall thickness1. Maintenance of LV systolic wall stress via development of LV hypertrophy is realised at the cost of impaired coronary circulatory function. This is related to the unique pathophysiologic conditions of severe AS affecting the coronary circulation from within the LV cavity as well as from the compromised coronary inflow. Hence, the coronary circulation is negatively affected by elevated myocardial compressive forces throughout the cardiac cycle, by rarefied myocardial vessels of the hypertrophied LV with prolonged oxygen diffusion distances, by weakened coronary forward compressive forces2, and by disturbed LV filling properties with prolonged relaxation.
As a consequence, myocardial ischaemia manifesting as angina pectoris is found in up to 30-40% of patients with AS irrespective of obstructive coronary artery disease (CAD), which is a prognostically ominous sign. Most commonly, ischaemia has been documented as reduced coronary flow reserve (CFR). Following surgical aortic valve replacement, CFR has been consistently found to be improved though not restored to normal values3. Transcatheter aortic valve implantation (TAVI) represents a unique model to study the immediate effect of afterload reduction in severe AS on the coronary circulation. Investigations published so far on the instantaneous coronary circulatory effect of TAVI-induced afterload reduction in severe AS have yielded inconsistent results2,4-8.
The purpose of the present investigation in patients with severe AS was to test the hypotheses that, in response to the acute afterload reduction by TAVI, CFR and fractional flow reserve (FFR), as well as coronary collateral flow index (CFI), increase acutely.
Methods
STUDY DESIGN AND PATIENTS
This was a prospective, observational, short-term longitudinal pathophysiologic investigation in 40 patients undergoing clinically indicated TAVI for severe aortic valve stenosis. The primary study endpoints were coronary flow reserve (CFR, s/s) and fractional flow reserve (FFR, mmHg/mmHg), as obtained by coronary mean transit time measurements for CFR (Figure 1), and by coronary pressure measurements for FFR (Figure 2). Secondary study endpoints were coronary collateral flow index (CFI, mmHg/mmHg), and true index of microcirculatory resistance (IMRtrue, mmHg s). Study endpoints were obtained immediately before and after TAVI. Eligibility criteria for study inclusion were age >18 years, severe aortic valve stenosis as indication for TAVI, and written informed consent to participate in the study. Exclusion criteria were acute coronary syndrome, and previous myocardial infarction in the vascular region undergoing endpoint measurement. The study was approved by the ethics committee of the Canton of Bern, Switzerland, and all patients gave written informed consent to participate.
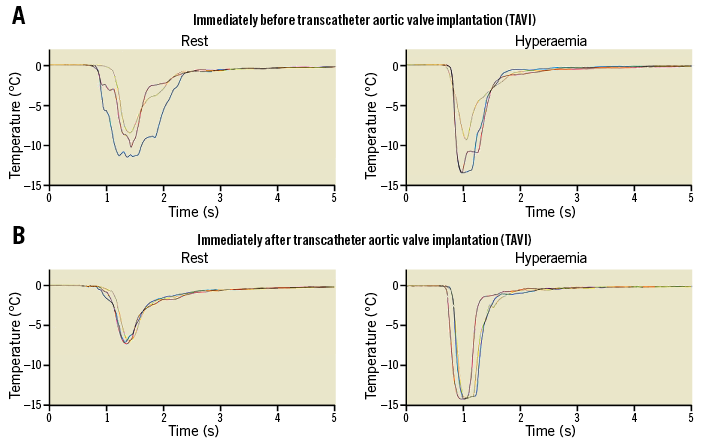
Figure 1. Coronary thermodilution measurements. A) Measurement of coronary, thermodilution-derived mean transit time before TAVI at rest (left side) and during hyperaemia (right side). Coronary blood temperature (vertical axis) is recorded over time (horizontal axis) by the angioplasty guidewire temperature sensor following the injection of three boluses of cold saline (different colours of lines). Mean transit times at rest are 0.57, 0.53 and 0.61 s (average: 0.57 s); mean transit times during hyperaemia are 0.22, 0.25 and 0.31 s (average: 0.26 s); CFR=0.57/0.26=2.19. B) Identical recordings to those in panel A immediately after TAVI. Mean transit times at rest are 0.59, 0.66, 0.67 s (average 0.64 s); during hyperaemia 0.21, 0.21, 0.22 s (average: 0.21 s); CFR=0.64/0.21=3.04. CFR: coronary flow reserve; TAVI: transcatheter aortic valve implantation
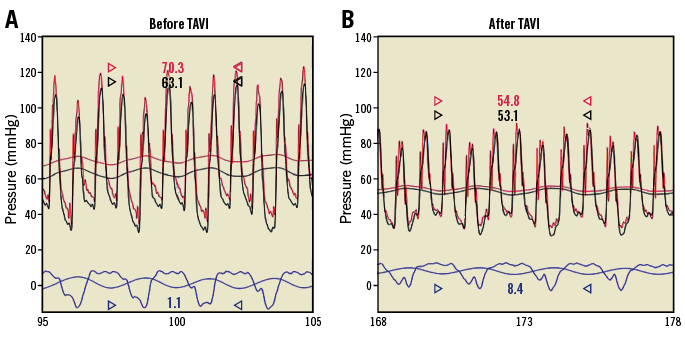
Figure 2. Fractional flow reserve measurements. A) Simultaneous recordings over time (horizontal axis) of phasic and mean aortic (vertical axis; red line), distal coronary (black line) and central venous pressure (blue line) before TAVI. FFR during i.v. adenosine at 140 g/kg/min in this case was 63.1/70.3=0.90. B) Identical pressure recordings to those in panel A after TAVI. FFR after TAVI was 53.1/54.8=0.97. FFR: fractional flow reserve; TAVI: transcatheter aortic valve implantation
CARDIAC CATHETERISATION AND CORONARY ANGIOGRAPHY
Patients underwent left and right heart catheterisation and coronary angiography for diagnostic purposes from the right femoral artery approach via a 6 Fr introducer sheath. Biplanar left ventriculography with recording of the pressure gradient across the aortic valve by pullback was performed followed by coronary angiography. Coronary artery stenoses were assessed quantitatively as percent lumen diameter reduction using the guiding catheter for calibration. Aortic pressure (Pao) was acquired via a 6 Fr guiding catheter. Central venous pressure (CVP) was measured by a 5 Fr pigtail catheter as right atrial pressure via the right femoral vein.
STUDY ENDPOINTS
All study endpoints were acquired immediately before and immediately following TAVI, and without altering the status of the coronary artery undergoing endpoint measurements. They were taken during resting conditions and, immediately thereafter, under conditions of myocardial hyperaemia as induced by intravenous adenosine at an infusion rate of 140 g/min/kg.
PRIMARY STUDY ENDPOINTS
CFR and FFR were obtained using a combined thermodilution/pressure sensor coronary angioplasty guidewire (PressureWire™ Certus™; St. Jude Medical, St. Paul, MN, USA) positioned distally in a stenotic coronary artery (among patients with CAD) selected for subsequent percutaneous coronary angioplasty (PCI), or in a normal coronary artery easily accessible for endpoint measurements. CFR was determined using the mean transit circulation time (Tmn, s) of a coronary cold bolus (Figure 1) injected at a volume of 3-4 ml of saline 0.9% at room temperature: CFR=hyperaemic Tmn–1 divided by resting Tmn–1=resting Tmn/hyperaemic Tmn. Simultaneously, coronary pressure-derived FFR was obtained as the ratio of hyperaemic mean distal coronary pressure (Pd) to mean aortic pressure (Pao) (Figure 2).
SECONDARY STUDY ENDPOINTS
Coronary occlusive collateral flow relative to normal antegrade flow through the non-occluded coronary artery (collateral flow index [CFI]) was determined using coronary pressure measurements. The 0.014-inch pressure monitoring angioplasty guidewire (PressureWire Certus) was set at zero, calibrated, advanced through the guiding catheter, and positioned in the distal part of the vessel of interest. CFI was determined by simultaneous measurement of Pao, the mean distal coronary artery pressure during balloon occlusion (Poccl, mmHg), and the mean central venous pressure (CVP, mmHg) as measured during the last 30 seconds of the one-minute coronary balloon occlusion. CFI was calculated as (Poccl–CVP) divided by (Pao–CVP)9. True index of microcirculatory resistance (IMRtrue, mmHg·s) was calculated as follows:
IMRtrue=Pao·Tmn·[(Pd–Poccl)/(Pao–Poccl)].
AORTIC VALVE STENOSIS AND TAVI PROCEDURE
Before TAVI, transthoracic and transoesophageal echocardiography was performed for severity assessment of aortic valve stenosis. Doppler-derived pressure gradients across the stenotic aortic valve as well as continuity equation-based valvular orifice area were measured. The TAVI procedures were performed with the balloon-expandable Edwards SAPIEN/SAPIEN XT bioprosthesis (Edwards Lifesciences, Irvine, CA, USA) or the self-expanding Medtronic CoreValve® bioprosthesis (Medtronic, Minneapolis, MN, USA) at the discretion of the operator. All of the TAVI procedures were performed without general anaesthesia and under conscious sedation with local anaesthesia for percutaneous access.
STUDY PROTOCOL
Following right and left heart catheterisation and diagnostic coronary angiography, all patients received 5,000 units of heparin intravenously at the start of the invasive study procedure. Endpoint measurements were first obtained under resting conditions in the coronary artery of interest. To that effect, the thermistor and pressure sensor guidewire was placed downstream in the non-occluded vessel of interest. For Tmn measurements, at least three coronary bolus injections of saline at room temperature were performed, and repeated in case the time values were beyond a 20% variability range. Proximal coronary angioplasty balloon occlusion with an unchanged sensor guidewire position was then performed for exactly one minute in order to acquire simultaneous pressure recordings (Pao, Poccl, CVP) for CFI. Complete coronary occlusion was ascertained by angiography. Tmn and pressure measurements were then repeated under intravenous adenosine at an infusion rate of 140 g/min/kg. The equipment for study endpoint measurements was removed, and patients underwent the TAVI procedure. Immediately afterwards, the above study endpoint measurements were repeated with the sensor guidewire located at the identical coronary arterial position. At the end of the procedure, left ventricular end-diastolic pressure and the pressure gradient across the newly implanted aortic valve prosthesis were determined. PCI of coronary arterial stenotic lesions was postponed until after completion of the study measurements.
STATISTICAL ANALYSIS
For the purpose of data presentation, two study groups were established based on the absence or presence of percent diameter coronary artery stenosis ≤ or >50% as estimated visually. An intra-individual comparison of CFR, FFR, IMRtrue, CFI and other continuous study parameters obtained immediately before and after TAVI was performed by a paired Student’s t-test. Between-group comparison of study endpoints and other continuous demographic, clinical, angiographic, and haemodynamic variables was performed using the unpaired Student’s t-test. A chi² test was used for comparison of categorical variables among the study groups. Continuous variables are given as mean and standard deviation.
Results
Twenty-six patients belonged to the group of patients with CAD as defined by the presence of at least one coronary artery stenosis with a percent diameter luminal narrowing of >50%; 14 patients had no CAD according to that definition.
PATIENT CHARACTERISTICS AND CLINICAL DATA AT BASELINE
There were no statistically significant differences between the groups regarding age (on average 80±9 years), gender, body mass index, the occurrence of angina pectoris, history of prior myocardial infarction, and cardiovascular risk factor prevalence (38/40 with hypertension). Patients in the CAD group were more often under treatment with platelet inhibitors than patients without CAD. Otherwise, there was no difference between the groups regarding cardiovascular medication. During the TAVI procedure, more than half the patients in both groups received intravenous vasopressor/inotropic agents (24/40 patients), in the vast majority noradrenaline.
HAEMODYNAMIC AND CORONARY CIRCULATORY DATA AT BASELINE
There were no statistical differences between the groups at baseline in heart rate, mean arterial blood pressure, left ventricular ejection fraction, mean central venous pressure, and the coronary artery selected for endpoint measurements (Table 1). The number of coronary arteries affected by CAD was higher in the group with than in that without CAD, and the percent diameter narrowing was higher in the group with CAD.
HAEMODYNAMIC AND CORONARY CIRCULATORY DATA RELATED TO TAVI
Aortic valve area by transthoracic echocardiography was <1 cm2 in both groups (Table 1). The invasively obtained mean pressure gradient across the stenotic aortic valve was lower in patients with than in those without CAD (Table 1). The mean aortic valve pressure gradient dropped to values below 10 mmHg in both groups in response to TAVI. Overall, LV end-diastolic pressure dropped from 18±7 mmHg before TAVI to 15±7 mmHg immediately after TAVI (p=0.0560). The absence of or the presence of mild aortic regurgitation as assessed by echocardiography the day following TAVI was 21/40 and 19/40, respectively (p=0.77) (Table 1).
PRIMARY STUDY ENDPOINTS
Overall, CFR was 1.9±0.9 before TAVI and 2.0±1.0 immediately after TAVI (p=0.72) (Figure 3). Resting mean transit time changed from 0.75±0.48 s before to 0.86±0.47 s after TAVI (p=0.0248) (Figure 3); hyperaemic mean transit time changed from 0.44±0.35 s before to 0.48±0.30 s after TAVI (p=0.53). The TAVI-induced, insignificant change in CFR did not differ between the groups with and without CAD (Table 1).
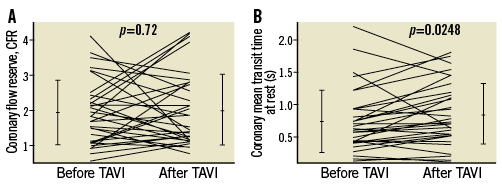
Figure 3. Coronary flow reserve measurements. A) Individual values of thermodilution-derived coronary flow reserve (CFR, vertical axis; coronary mean transit time at rest/mean transit time during hyperaemia) before and after TAVI. B) Individual values of coronary mean transit time under resting conditions (vertical axis) before and after TAVI. Error lines indicate mean values±standard deviation. TAVI: transcatheter aortic valve implantation
There was no significant difference in FFR between the groups either before or after TAVI (Table 1). In the entire study population, FFR increased from 0.90±0.08 before TAVI to 0.93±0.08 after TAVI (p=0.0021) (Figure 4). The TAVI-induced increase in FFR was related to a significant decrease in hyperaemic mean aortic pressure (Pao) from 71±16 mmHg before TAVI to 67±15 mmHg after TAVI (p=0.009) (Figure 4); the respective values of Pao did not differ significantly between the groups either before or after TAVI (Table 1). Hyperaemic mean distal coronary pressure (Pd) remained statistically constant before and after TAVI (Figure 4).
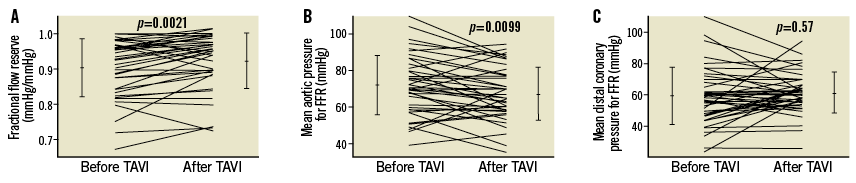
Figure 4. Coronary and aortic haemodynamic pressure measurements. A) Individual values of FFR (vertical axis) before and after TAVI. B) Individual values of mean aortic pressure as obtained during myocardial hyperaemia for FFR (vertical axis) before and after TAVI. C) Individual values of mean distal coronary pressure as obtained during hyperaemia for FFR (vertical axis) before and after TAVI. Error lines indicate mean values±standard deviation. FFR: fractional flow reserve; TAVI: transcatheter aortic valve implantation
SECONDARY STUDY ENDPOINTS
In the entire study population, CFI under resting conditions decreased from 0.149±0.080 before TAVI to 0.126±0.076 immediately after TAVI (p=0.0553) (Figure 5). The change was due to a significant increase in mean CVP from 8.6±3.9 mmHg before TAVI to 9.9±3.6 after TAVI (p=0.0020). Hyperaemic CFI increased from 0.127±0.083 before to 0.146±0.090 after TAVI (p=0.0508) (Figure 5).
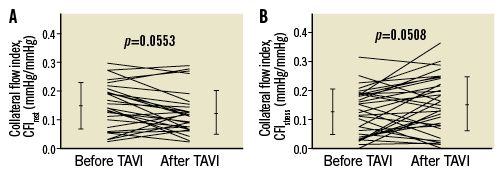
Figure 5. Coronary collateral flow index measurements. Individual values of coronary CFI (vertical axes) as obtained before and after TAVI during resting conditions (CFIrest, panel A) and during hyperaemia (CFIstress, panel B). Error lines indicate mean values±standard deviation. CFI: collateral flow index
Overall, IMRtrue during myocardial hyperaemia changed from 26.6±18.3 mmHg s before to 30.7±21.7 mmHg s after TAVI (p=0.42).
Discussion
CORONARY FLOW RESERVE RESPONSE TO TAVI
It is undisputed that the capacity to augment coronary flow under hyperaemic conditions is compromised, and thus CFR is decreased in severe AS even in the absence of epicardial coronary stenoses10,11. This result from the literature was corroborated by the present investigation, i.e., mean coronary transit time-based CFR was on average 1.9 at baseline exam. Following surgical aortic valve replacement, CFR has been consistently reported to be restored to normal values3. This CFR augmentation representing a midterm result following surgical aortic valve replacement has recently been confirmed as a short-term coronary circulatory response to TAVI2,4,6,7; therefore, it appeared justified to hypothesise a CFR increase instantly following TAVI for the present investigation. The main difference to CFR as determined actually consisted in the fact that it has been coronary velocity-derived in the previous investigations, whereas in the present one it was coronary transit time-based. Mean coronary transit time by cold bolus injection is inversely related to coronary flow and, as such, CFR is equal to 1/Tmn_stress÷1/Tmn_rest, which is equal to Tmn_rest÷Tmn_stress. Tmn_rest showed an increase in response to TAVI from 0.75±0.48 s before to 0.86±0.47 s (p=0.02) (Figure 3), and thus coronary flow at rest decreased immediately following afterload reduction. As a consequence, CFR tended to increase at constant hyperaemic mean transit time (Tmn_stress), whereby this trend was not distinguishable from signal variability related to cold bolus measurements, and was therefore statistically irrelevant. In the particular context of severe AS, it must be suspected that Doppler-derived coronary flow velocity measurements are more robust than thermodilution measurements for determining CFR. Alternatively, the influence of variable stenosis severities in our group with CAD could have influenced the study results as CFR noise. Despite the fact that such a likely explanation might have contributed to the study results, it was not discernible statistically, and, in the group without CAD, CFR decreased rather than increased immediately after TAVI. Practically, the tendency of the guiding catheter to being pushed out by the coronary bolus injection had to be closely watched and sometimes counteracted by choosing a different guiding catheter providing more support; an unnoticed action of such a quality would certainly have introduced considerable data variability. The calculation of the index of microcirculatory resistance is qualitatively influenced by identical pitfalls as it is also based on coronary mean transit time.
FRACTIONAL FLOW RESERVE RESPONSE TO TAVI
Whenever present, coronary stenotic lesions were not treated by PCI in our study between the two coronary haemodynamic measurements immediately before and after LV afterload reduction. Thus, any changes in FFR in response to TAVI must have been due either to microcirculatory or to collateral function changes or to both, because FFR is not a pure indicator of haemodynamic stenosis severity but a myocardial index which, in addition, is influenced by coronary collateral function: FFR=CFI+FFRstenosis12. Given the fact that the hyperaemic index of microcirculatory resistance remained constant before and after TAVI, it cannot be held responsible for the consistent increase in FFR observed before and after TAVI. Biologically, a microcirculatory-induced increase in FFR would not have made much sense anyway, because – under the conditions of identical coronary stenosis severity and constant CFI – it would have meant heightened microcirculatory resistance, which would have been counterintuitive considering the lowered myocardial transmural pressure in the context of reduced LV filling pressure. In the literature, FFR measurements immediately pre and post TAVI have been documented only rarely, and different trends have been observed depending on the FFR before TAVI below or above the haemodynamically relevant threshold of 0.88. That is to say, in the group of coronary stenotic lesions (n=21) with an FFR ≤0.80 before TAVI, it decreased in response to TAVI from 0.71 to 0.668; conversely, Pesarini et al described a significant FFR increase from 0.92±0.06 to 0.93±0.07 in response to TAVI among the group of coronary lesions with a baseline FFR >0.808. The latter result almost exactly corresponds to the findings of our study. The result of similar FFR values among patients with and without coronary stenosis in the vessel of interest is surprising at first sight, but most likely relates to the fact that it was treated by PCI in some patients before the study measurements. Importantly, however, PCI was not carried out in between the coronary study measurements in any of the patients. As Figure 4 illustrates, it is the consistent aortic pressure decrease in response to TAVI which is responsible for the augmented FFR, a fact which is explained by the effect of this procedure on the elevated sympathetic tone prevalent among patients with severe aortic stenosis13.
CORONARY COLLATERAL FUNCTION RESPONSE TO TAVI
As a consequence of the above-described FFR results with an increase immediately following LV afterload reduction at constant coronary microcirculatory resistance, the augmentation has to be reflected by improved collateral function during myocardial hyperaemia. At rest, a trend to decreased CFI was present, which was probably due to a significant central venous pressure augmentation in the context of peri-interventional fluid management. Despite an ongoing trend to increased CVP during hyperaemia, the tendency to an increase in coronary occlusive pressure and a decrease in mean aortic pressure had a net effect of augmenting CFI in response to TAVI. Biologically, this behaviour of collateral function improvement is reasonable considering the supreme relevance of the early diastolic suction period on coronary circulatory function. From a coronary wave intensity perspective, the energy under resting conditions during early diastolic suction in severe aortic stenosis is augmented, a fact which is interpreted as a compensatory mechanism in the presence of diastolic dysfunction5. During tachycardia, early diastolic suction wave energy falls in the presence of severe AS, and the described pattern at rest and during tachycardia is restored to the normal, lower resting and enhanced stress wave energy amplitude following afterload reduction by TAVI5. Accordingly, CFI as one aspect of coronary circulatory function is augmented during hyperaemia as an expression of enhanced early diastolic suction. That in our study the augmented early diastolic suction wave energy did not beneficially manifest in all comprehensive coronary haemodynamics is puzzling, but may be best explained with insufficient robustness of transit time as opposed to coronary pressure measurements.
Study limitations
The factor most severely influencing the study results was the pharmacological augmentation of afterload using noradrenaline. This substance was preferably used in 60% of the patients during the TAVI procedure in order to support systemic arterial pressure. It had been employed for the specific reason of counterbalancing the hypotonic effect of systemic adenosine, which was used for steady state hyperaemia induction crucial for thermodilution measurements. Since inotropes were employed with similar frequency pre and immediately post TAVI, the individual effect on study endpoints can be considered similar. The setting of coronary haemodynamic measurements immediately pre and post TAVI has been welcomed as the most physiologic in regard to observing the acute effects of LV afterload reduction14. This is certainly the case when comparing the actual situation to TAVI under general anaesthesia with preload and afterload changes in the context of, e.g., mechanical ventilation. However, part of the conflicting evidence of acute afterload reduction affecting the coronary circulation in severe AS is due to variably employed vasoactive substances. In this context, myocardial hyperaemia induction by means other than systemic adenosine, e.g., by coronary regional reactive hyperaemia in response to brief coronary balloon occlusion, might have reduced the use of circulatory supportive measures interfering with a haemodynamic situation resembling the normal physiologic state12. A sub-analysis of the data according to the absence (n=14) or presence (n=26) of vasopressor support did not yield endpoint results in response to TAVI which were different from the entire population. As a further limitation, the present work did not obtain long-term but only instantaneous coronary haemodynamic effects of LV unloading by TAVI.
Conclusions
CFR and microcirculatory resistance appear not to be acutely affected by LV afterload reduction in severe AS in response to TAVI. However, it acutely improves FFR independent of interfering treatment of coronary stenoses; this occurs via lowering of mean aortic pressure. Hyperaemic coronary collateral flow index tends to augment in response to TAVI.
| Impact on daily practice The haemodynamic severity of mild coronary stenoses is mitigated immediately following TAVI for severe AS, suggesting that PCI of these lesions may not be indicated. |
Acknowledgements
We thank Raphael Grossenbacher, RN, Hélène Steck, RN, Fabian Praz, MD, and Reto Kurmann, MD, for their valuable work in patient recruitment and expert technical assistance during data acquisition.
Funding
This study was supported by a grant from the Swiss National Science Foundation for research (grant #3200B_163256/1 to CS) and from the Swiss Heart Foundation (to SG and CS).
Conflict of interest statement
The authors have no conflicts of interest to declare.

