Aortic stenosis (AS) induces left ventricular (LV) hypertrophy by imposing a pressure load at the valve level. Haemodynamic stress along with increased mass changes the density of the myocardial capillary bed and resistances to myocardial flow; at times, this may result in a mismatch of myocardial oxygen supply/demand, which may explain inducible ischaemia despite normal coronary arteries. Relief of the valvular stenosis should, in theory, ameliorate such myocardial changes over time, resulting in improved LV function, reduced LV mass, and better coronary physiology, manifesting in an improved coronary flow reserve (CFR) (Figure 1). Envisioning this response is easy, proving it is hard.
In this issue of EuroIntervention, to this point, Sabbah and colleagues1 examined data from 34 patients with aortic valve replacement (AVR) (17 with transcatheter AVR, 14 with surgical bioprosthetic AVR, and 3 with surgical mechanical valves). Each patient underwent thermodilution coronary flow measurement in the left anterior descending (LAD) artery before and 6 months after AVR, at rest and during hyperaemia. They found that CFR (the ratio of hyperaemic/basal flow) increased from a median of 2.5 (1.5-3.3) at baseline to 3.1 (2.2-5.1) at follow-up (p=0.005). This improvement in CFR occurred without any appreciable change in maximal hyperaemic flow (QLAD; 230±106 mL/min to 250±101 mL/min; p=0.26) or minimal microvascular resistance (Rμ, LAD; 347 [247-463] to 287 [230-456]; p=0.20). Moreover, there were no changes in FFR or the index of microvascular resistance (IMR). Contrary to common belief, the increases in CFR were due entirely to reductions in basal flow (measured by a longer resting mean transit time [Tmn] of 0.51±0.38 vs 0.71±0.5) with a stable hyperaemic mean transit time. In other words, basal flow fell while hyperaemic flow remained the same, yielding an increased CFR.
The authors went further, providing correlated data on changes in LV mass by cardiac magnetic resonance imaging (cMRI) before and 6 months after AVR. They found that AVR causes a 20% reduction in LV mass index and concentric hypertrophy, and a reduction in LV stroke work. The absolute reduction in the LV stroke work index (but not the LV mass index) was statistically correlated with an increase in CFR (r= –0.40; p=0.041). Paradoxically, when analysed indexed to LV mass, hyperaemic flow increases, whereas resting flow is unchanged, a reversal of the non-indexed comparison. Which is more valid remains up for discussion but does fuel the fire of controversy.
Limitations of this study are few but include the large variability of individual changes seen after AVR. Half of the patients did not have LV hypertrophy at baseline despite having severe AS, creating some question as to the validity of the LV mass change results. CFR measurements using bolus thermodilution flow are also prone to variability. Continuous thermodilution flow or Doppler measurements of resistance and CFR may have produced different results.
Should these results influence our thinking about the use of translesional coronary haemodynamics before and after AVR? By its fundamental design, FFR is known to be less susceptible to changes in heart rate, contractility, and blood pressure than CFR. FFR is specific to the epicardial artery and much less influenced by the microcirculation, provided it is stable. Both FFR and CFR change after acute coronary syndromes, as microvascular function and myocardial flow recuperate over a period of weeks. The situation is different for AS, as changes in myocardial mass, loading conditions, and microvascular capillary density develop over years and may take months to regress if such a change occurs at all.
The authors are to be commended for a detailed study, extending their previous work and providing new insights. They demonstrate that although CFR increases after AVR, the reason is not because of increasing maximal hyperaemic flow or reductions in microvascular resistance from reduced LV mass, but because resting flow and stroke work have decreased in response to reduced myocardial demand. The authors suggest that this finding validates their previous study indicating that FFR is not significantly changed after transcatheter AVR2 and can thus be relied upon even in the setting of severe aortic stenosis. In the same study, RFR (the resting flow translesional pressure ratio) changed significantly from 0.88 (0.83%–0.93) at baseline to 0.92 (0.83–0.95) at follow-up (p=0.003)2. In contrast, Ahmad et al noted that hyperaemic coronary flow (by Doppler) but not resting flow during the wave-free period increased significantly post-TAVR, suggesting that compared to FFR, non-hyperaemic pressure ratios (NHPR), e.g., RFR, are less impacted by AS3. The conflicting studies likely reflect multiple factors involved in both the clinical substrate of AS patients as well as methodological considerations. The quest to understand AS and AVR and their relationship to myocardial blood flow, resistance, and reserve continues.
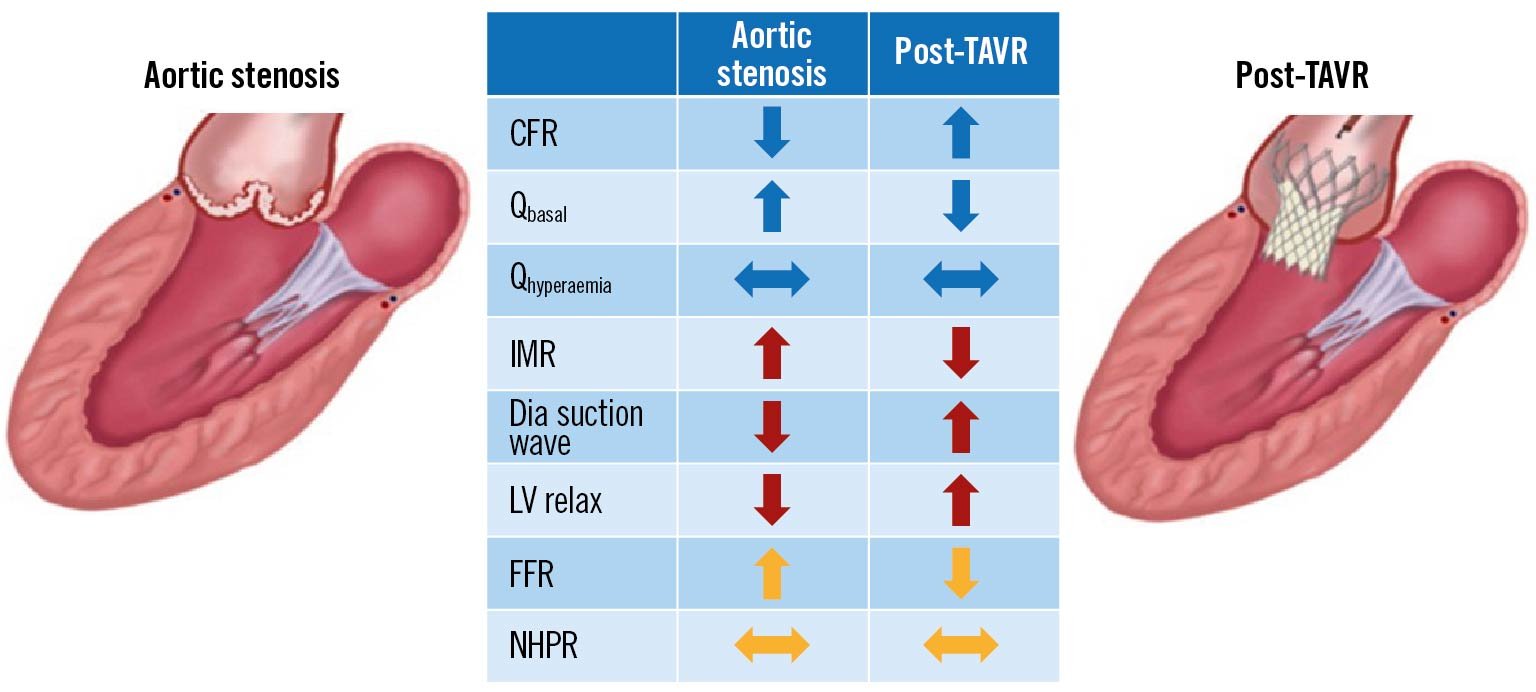
Figure 1. Changes in coronary haemodynamic parameters before and after aortic valve replacement including coronary flow reserve (CFR), resting flow (Qbasal), hyperaemic flow (Qhyperaemia), index of microvascular resistance (IMR), diastolic (Dia) suction wave, left ventricular relaxation (LV relax), fractional flow reserve (FFR), and non-hyperaemic pressure ratio (NHPR). Arrows indicate increase, decrease or no change. Modified with permission from6.
The bottom line
Regardless of the mechanisms around CFR, from a practical standpoint the need for physiologic coronary assessment prior to AVR may be declining. For those patients with planned surgical AVR with coronary artery bypass surgery, any benefit of physiologic assessment remains elusive4. For those patients with planned transcatheter AVR and percutaneous coronary intervention (PCI), the recent ACTIVATION trial has demonstrated no benefit and more bleeding for PCI prior to AVR5. Patients with coronary disease and severe aortic stenosis can thus proceed with transcatheter AVR and plan to have physiologically-guided PCI afterwards. Nevertheless, in-depth studies like that of Sabbah et al1 challenge conventional wisdom and physiological assumptions, directing us toward new avenues of research and practice.
Conflict of interest statement
M.J. Kern is a speaker for Abbott Vascular, Boston Scientific, ACIST Inc, and OpSens Inc. A.H. Seto has research grants from Philips and ACIST; honoraria from Getinge, General Electric, and Terumo, and equity in Frond Medical.

