Abstract
Aims: The aim of this study was to investigate the diagnostic performance of quantitative flow ratio (QFR) in assessing the physiological relevance of coronary lesions in the presence of severe aortic valve stenosis (SAS).
Methods and results: A total of 115 SAS patients (138 coronary arteries) were included. Functional assessment of coronary stenoses was performed with fractional flow reserve (FFR) before transcatheter aortic valve implantation (TAVI). Subsequently, QFR was calculated at a central core laboratory, blinded to FFR results. The diagnostic yield of QFR was assessed using FFR as reference. Coronary stenoses were intermediate (diameter stenosis 48±10%, FFR 0.84 [0.77-0.89], QFR 0.82 [0.73-0.89]). Per-vessel sensitivity, specificity, area under the ROC curve and accuracy of QFR were 84% (95% CI: 71-92%), 80% (95% CI: 69-88%), 0.88 (95% CI: 0.82-0.93) and 81%, respectively. Diagnostic accuracy of QFR decreased significantly in patients with aortic valve area (AVA) <0.60 cm2. Diagnostic performance of QFR was superior to angiography in assessing the FFR-based functional significance (AUC 0.88 [95% CI: 0.82-0.93] vs 0.74 [95% CI: 0.66-0.81], respectively; p=0.0002).
Conclusions: Compared with FFR, QFR has a good diagnostic yield and is superior to angiography in assessing the functional relevance of coronary lesions in SAS patients awaiting TAVI, particularly when AVA is ≥0.6 cm2.
Introduction
Concomitant coronary artery disease (CAD) is frequently found in patients with symptomatic severe aortic valve stenosis (SAS) undergoing transcatheter aortic valve implantation (TAVI), making their management particularly challenging1. In this clinical setting, coronary revascularisation is empirically recommended in proximal and angiographically severe coronary stenoses2. Although functional stenosis evaluation with fractional flow reserve (FFR) or instantaneous wave-free ratio (iFR) has been shown to improve cardiovascular outcomes in patients with stable CAD2, the use of these indices in patients with SAS is hampered by substantial differences in coronary physiology and patient characteristics. Both indices can be affected by altered left ventricular pressures, microcirculatory function, and the development of left ventricular hypertrophy caused by SAS3,4,5. Despite some studies suggesting that, even in the presence of SAS, FFR can be used to assess coronary stenoses4,6,7, larger studies are required to confirm these findings. However, even if the reliability of FFR in this setting is confirmed with larger series, the haemodynamic frailty of patients undergoing TAVI will probably deter many operators from using vasoactive drugs and performing intracoronary wire assessment.
Recently, wire- and adenosine-free functional assessment of coronary stenoses has become possible due to developments in functional angiography, allowing calculation of virtual FFR based on computation of three-dimensional quantitative coronary angiography (3D-QCA) with mathematical or fluid dynamics-derived equations8. One of these methods, quantitative flow ratio (QFR)9, has shown a high accuracy in determining the physiological relevance of coronary stenoses in different clinical settings10,11,12. While the concept of QFR may be very attractive for guiding clinical decisions in SAS patients with concomitant CAD, its potential applicability in patients awaiting TAVI has never been investigated. The objective of this study was to determine the diagnostic yield of QFR in assessing the functional relevance of coronary stenoses in SAS patients before TAVI, using FFR assessment as the reference standard.
Methods
STUDY DESIGN AND POPULATION
The QASTA study (functional assessment of coronary stenoses by the novel quantitative flow ratio in patients with severe aortic stenosis undergoing transcatheter aortic valve implantation) is a multicentre, retrospective study with blind analysis of angiographic data involving seven centres from Spain, Italy, Canada, South Korea and the USA. The study enrolled patients with SAS and concomitant CAD in whom coronary stenoses were interrogated with FFR before TAVI. Coronary stenoses suitable for FFR interrogation were defined according to operator criteria, usually involving lesions with diameter stenosis between 40 and 80% by visual estimation. SAS was diagnosed by transthoracic echocardiography using the valve area (<1.0 cm2; indexed valve area <0.6 cm2/m2 body surface area) or flow-pressure parameters (mean gradient >40 mmHg, maximum jet velocity >4.0 m/s, and velocity ratio <0.25)13. DICOM files of coronary angiograms were sent to and centrally analysed in the QFR core laboratory located at Hospital Clinico San Carlos, Madrid, Spain. QFR assessment was performed by certified analysts, blinded to both FFR values and clinical decisions about coronary revascularisation. QFR analysis was performed using the routine diagnostic angiography acquired before TAVI. The diagnostic performance of QFR in determining the functional stenosis relevance was assessed using FFR before TAVI as reference. Exclusion criteria were ostial disease in the left main or in the right coronary artery, target vessel with collateral circulation or coronary flow from patent surgical grafts, severe diffuse disease, in-stent restenosis, target vessel with myocardial bridging, target vessel with previous myocardial infarction, poor angiography image quality, absence of two angiographic projections separated by more than 25º, angiograms with frame rate <12.5 frames per second, and severe tortuosity or overlapping limiting an optimal 3D reconstruction of the target vessel. Participants gave their written informed consent for the index physiological procedures and the study was conducted according to the Declaration of Helsinki.
INVASIVE FFR ASSESSMENT, QFR ANALYSIS AND STATISTICS
Invasive FFR assessment, QFR analysis and statistics are shown in Supplementary Appendix 1-Supplementary Appendix 3 and Supplementary Figure 1.
Results
BASELINE CHARACTERISTICS OF THE STUDY POPULATION
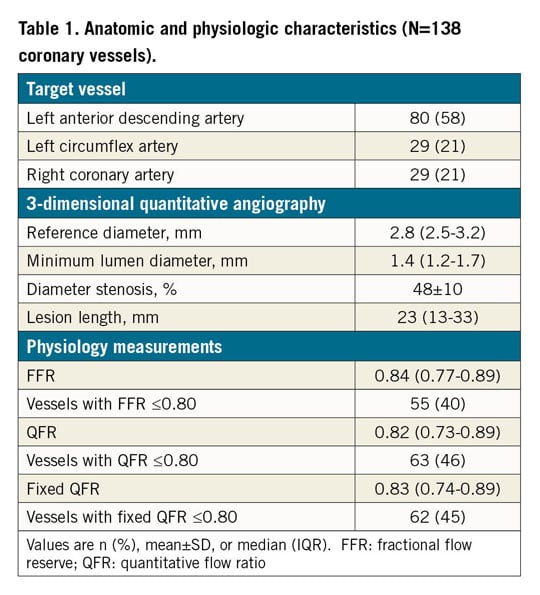
A total of 138 coronary arteries from 115 patients were included in the analysis after the participation criteria had been fulfilled (Figure 1). Median age was 82 years (IQR 75-86); 47% of the patients were male. Mean aortic pressure gradient and aortic valve area (AVA) were 47.5±16.9 mmHg and 0.68±0.22 cm2, respectively. The most commonly interrogated vessel was the left anterior descending coronary artery (58%), and the majority of target stenoses were of adequate size for percutaneous angioplasty (reference diameter 2.8 mm [IQR 2.5-3.2]). The clinical and anatomical characteristics of the study population are summarised in Table 1 and Supplementary Table 1.
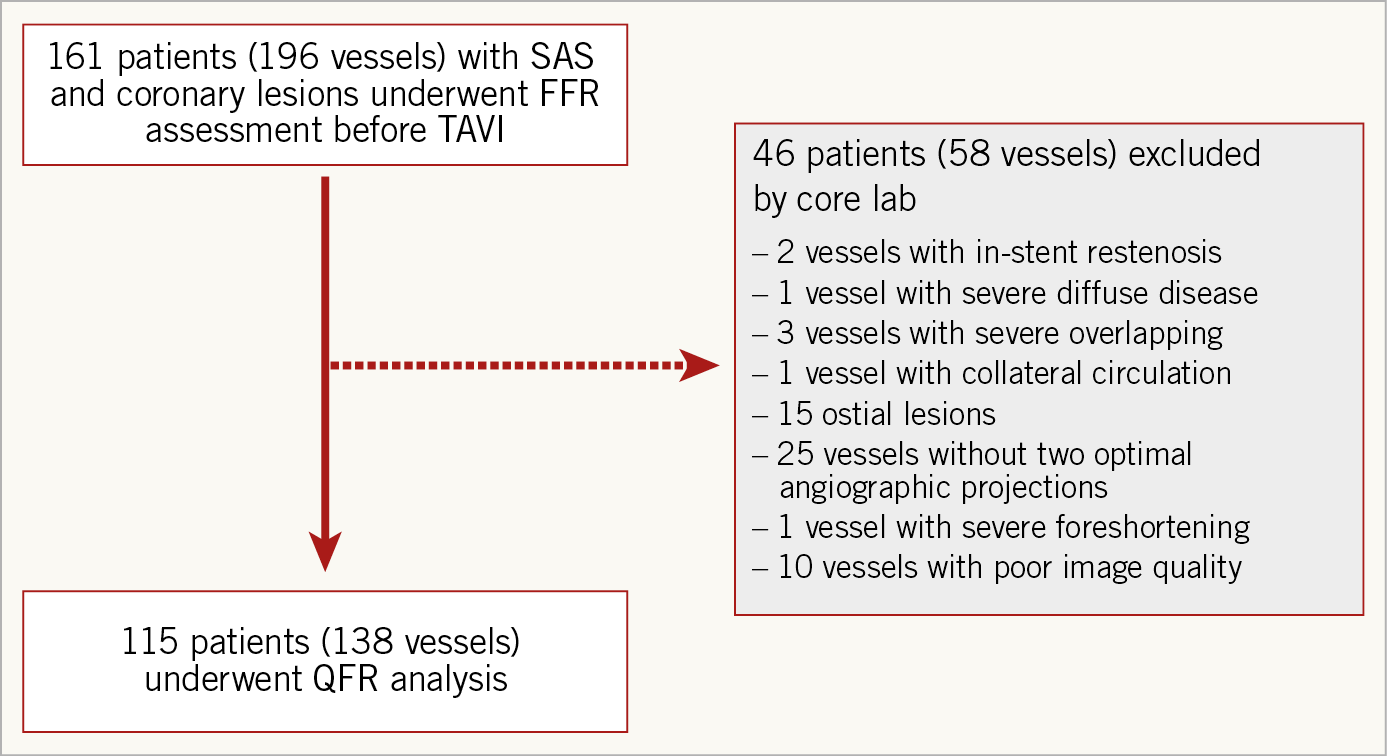
Figure 1. Study flow chart. FFR: fractional flow reserve; QFR: quantitative flow ratio; SAS: severe aortic stenosis; TAVI: transcatheter aortic valve implantation
CORONARY STENOSIS SEVERITY AND PHYSIOLOGICAL ASSESSMENT
In per-vessel analysis, overall stenosis severity was angiographically and functionally intermediate as determined by the percent diameter stenosis (48±10% derived from 3D-QCA), FFR (median 0.84 [0.77-0.89]) and QFR (median 0.82 [0.73-0.89]) (Figure 2, Table 1). The number of ischaemia-causing stenoses as judged by FFR ≤0.80 or QFR ≤0.80 was similar for both methods (40% vs 46%, respectively; p=0.315).
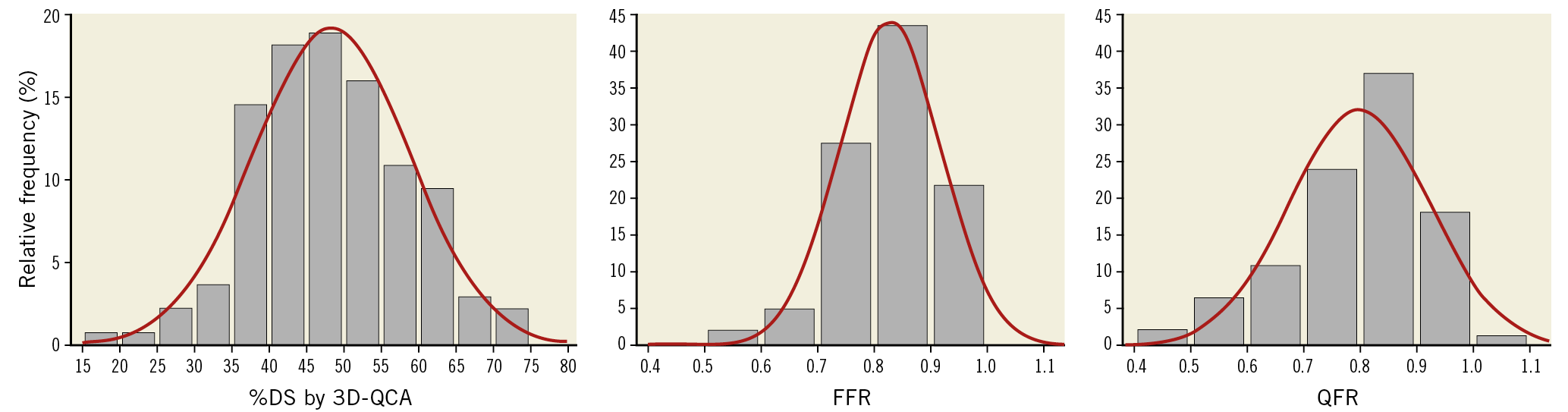
Figure 2. Coronary stenosis severity of the study population. Per-vessel analysis depicts intermediate severity of coronary stenoses as determined by %DS by 3D-QCA, FFR and QFR. FFR: fractional flow reserve; QFR: quantitative flow ratio
DIAGNOSTIC PERFORMANCE OF QFR
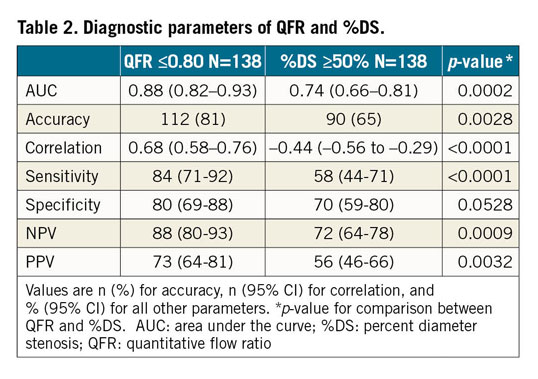
Supplementary Figure 2 shows the correlation and agreement between FFR and QFR. The per-vessel receiver operating characteristic (ROC) assessment using FFR ≤0.80 as the reference identified a sensitivity, specificity and area under the curve (AUC) of QFR of 84% (95% CI: 71-92), 80% (95% CI: 69-88) and 0.88 (95% CI: 0.82-0.93), respectively (Table 2). Using ≤0.80 as cut-off for both techniques, QFR correctly classified the functional significance of coronary stenosis in 112 vessels (81%).
COMPARISON BETWEEN QFR AND %DS IN PREDICTING FFR ≤0.80
QFR was superior to angiography (%DS by 3D-QCA) in determining the functional significance of coronary stenoses as assessed by per-vessel ROC analysis: AUC 0.88 versus 0.74 (p=0.0002 for ROC curve comparison) (Figure 3A), sensitivity 84% versus 58% (p<0.0001) and specificity 80% versus 70% (p=0.0528) (Table 2).
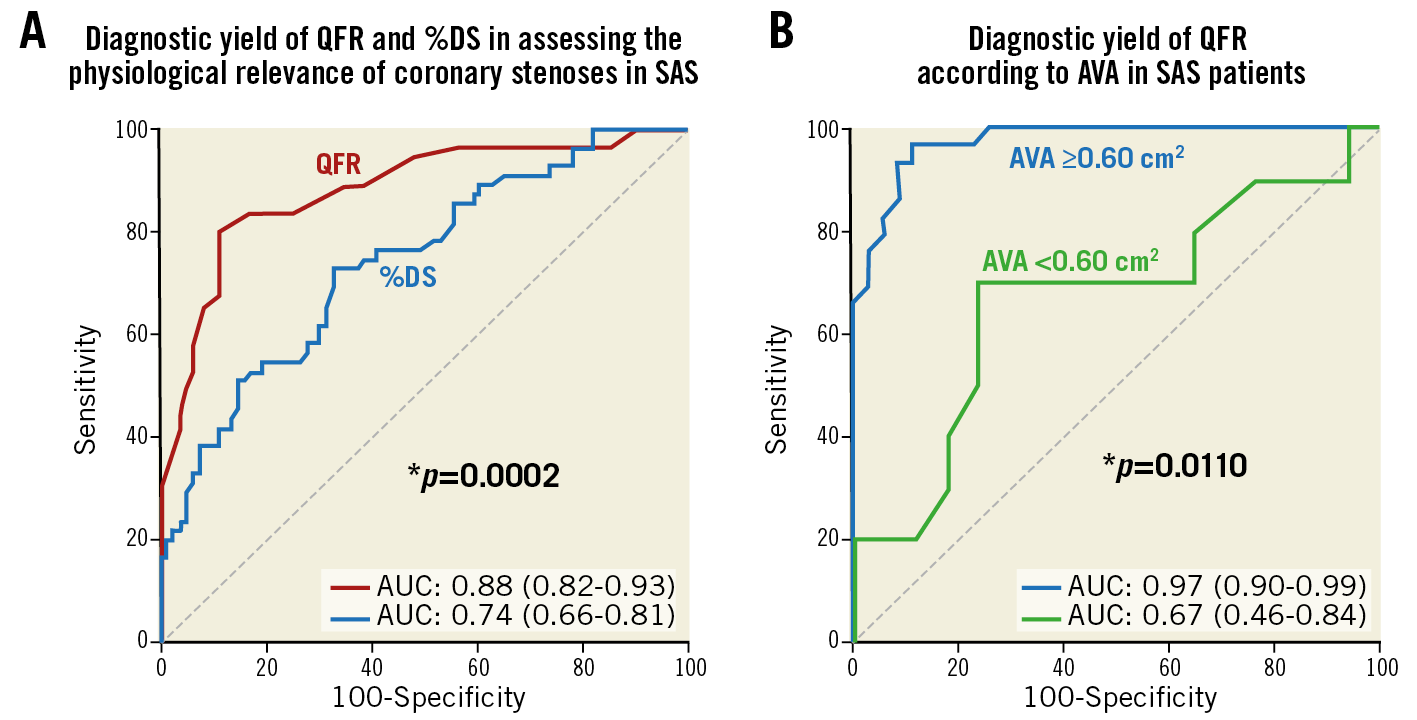
Figure 3. Diagnostic yield of QFR in SAS patients. A) Comparison of area under the curve (AUC) between QFR and percent diameter stenosis (%DS) using FFR ≤0.80 as reference. B) Diagnostic yield of QFR according to aortic valve area (AVA). *p-value for comparison of AUC.
ROC analysis identified ≥49% as the best cut-off for %DS (by 3D-QCA) to predict an FFR ≤0.80 (Supplementary Figure 3). Using the cut-off ≤0.80 for QFR and ≥50% for %DS, QFR was superior to angiography in correctly classifying FFR-based functional stenosis relevance (112 vessels [81%] vs 90 vessels [65%], respectively; p=0.0028). Per-patient ROC analysis is shown in Supplementary Figure 4.
EFFECT OF AORTIC STENOSIS SEVERITY ON QFR DIAGNOSTIC PERFORMANCE
The classification agreement between QFR and FFR was significantly different across different ranges of AVA (Figure 4A). In patients with an AVA ≥0.80 cm2, the classification agreement between both methods was as high as 91%, decreased to 79% when AVA was 0.60–0.80 cm2, and 66% when AVA was <0.60 cm2 (p=0.022 for comparison between AVA ranges). In addition, the AUC for QFR in patients with AVA ≥0.60 cm2 was as high as 0.97, whereas in patients with AVA <0.60 cm2 it decreased to 0.67 (p=0.0110 for comparison between AUC) (Figure 3B). A significant effect of aortic stenosis severity on the agreement between QFR and FFR could not be demonstrated when mean transvalvular pressure gradient was used as an index of reference (Figure 4B).
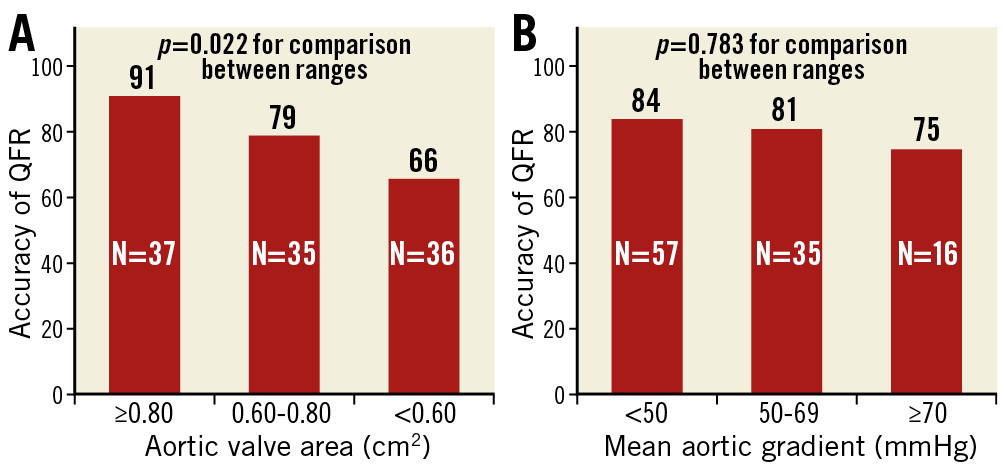
Figure 4. Accuracy of QFR according to aortic stenosis severity. A) Accuracy of QFR (i.e., classification agreement with FFR) decreased as aortic valve area decreased, being significantly lower in patients with aortic valve area <0.60 cm2. B) A trend towards lower accuracy of QFR was observed when mean aortic gradient increased. N: number of patients in each range of aortic valve area or mean aortic gradient
Discussion
The main findings of this study are the following. 1) Overall, QFR has a good diagnostic yield in determining the physiological relevance of coronary stenoses in SAS patients scheduled for TAVI, as estimated with FFR. 2) The diagnostic efficiency of QFR is particularly high (AUC 0.97) when patients with very severe aortic stenosis (AVA ≤0.60 cm2) are excluded. 3) Compared with angiography, QFR is superior in determining the FFR-based physiological stenosis relevance.
Up to 50% of patients with symptomatic SAS present concomitant CAD1,14. In that subset, current clinical practice guidelines recommend revascularisation if coronary stenosis involves proximal vessel segments and in patients in whom surgical valve replacement or TAVI is planned2. Of note, this recommendation is based on angiographic assessment of stenosis severity, whereas physiology-guided clinical decision making is not yet supported by the clinical guidelines. This is due to a number of considerations. 1) There is a paucity of data concerning the prognostic impact of CAD in SAS patients, especially in elderly patients. 2) The value of PCI before TAVI is questionable in elderly patients with moderate CAD, SAS and absence of angina symptoms. 3) Randomised clinical trials evaluating the clinical value of physiology-guided revascularisation in SAS patients have never been conducted. 4) Coronary haemodynamics secondary to structural and functional abnormalities caused by SAS may influence intracoronary trans-stenotic pressure gradients.
Notwithstanding this, numerous efforts have been made to understand coronary haemodynamics caused by aortic stenosis, and how treatment of aortic stenosis modifies physiological assessment of coronary lesions by pressure gradients. Some observational studies found that FFR values obtained under the challenging physiological conditions of SAS are similar to those obtained after TAVI4,6,7. However, despite promising results it remains to be seen whether wide adoption of FFR will occur in this population. Many operators may be reluctant to use vasoactive drugs (which are fundamental to performing FFR) in order to avoid potential haemodynamic adverse effects in the presence of SAS. Considerations that have been put forward to explain the relatively low adoption of FFR outwith the context of SAS, such as associated costs, increased use of contrast, and length of the diagnostic procedure, may also influence its use in patients with SAS.
To circumvent these challenges for functional coronary lesion assessment in SAS, we investigated the diagnostic yield of QFR, a novel technique based on fast computation of coronary angiography using advanced mathematical algorithms. Previous studies reported a high accuracy of QFR in different clinical settings, including stable CAD and non-culprit stenoses in acute coronary syndromes10,11,12,15, but its diagnostic yield in the presence of SAS has not been evaluated. In this study we demonstrated that, overall, QFR has a good diagnostic performance in determining the FFR-based functional relevance of coronary stenoses in patients awaiting TAVI, with an AUC of 0.88 and classification agreement of 81%. The applicability of our findings is supported by the characteristics of the study population, with mean values of physiological indices (FFR, QFR), angiography stenosis severity (%DS) and aortic valve severity similar to those reported in previous registries10,16,17. Furthermore, the use of FFR as a reference obtained before TAVI is supported by available studies4,7.
Our study also provides new insights into the impact of SAS on the functional assessment of coronary stenoses with pressure guidewires. Although we found an overall good diagnostic yield of QFR, it was lower compared with previous studies out of the context of aortic valve disease10,11. A plausible hypothesis for this is that the decrease in diagnostic accuracy is due to the presence of microvascular dysfunction, which in the case of SAS may be the result of numerous mechanisms including structural remodelling of microvessels, increased extravascular compression of capillaries due to left ventricular pressure overload, and left ventricular hypertrophy1,5,18,19. In theory, a graded increase in these microvascular abnormalities could be expected as the severity of aortic stenosis increases3, modifying the correlation and agreement between QFR (an angiography-based method that partially ignores the microcirculatory status) and FFR (an intracoronary technique subject to modulation by the subtended microcirculation). This could also explain why the QFR diagnostic performance decreased as AVA decreased. Therefore, we hypothesise that the discrepancy between QFR and FFR in SAS should reflect the extent to which the microcirculatory status deviates from the expected reference status (i.e., the boundary conditions assumed in QFR calculation for healthy coronary circulation). In support of this rationale, a previous study from our group found a lower diagnostic performance of QFR compared with FFR in the presence of high microvascular resistance20. However, because in our study we do not have invasive coronary flow measurements, coronary flow reserve (CFR) or microvascular resistances cannot be derived, and our reasoning can be considered only as hypothesis-generating.
Abnormalities in coronary flow also deserve attention as potential contributors to discrepancies between QFR and FFR. A number of studies have demonstrated that resting coronary flow is significantly increased in SAS patients, being the main factor affecting CFR as a consequence of reduced delta between hyperaemic and resting flow5,21. It is important to note that algorithms for calculation of a contrast-QFR model estimate the hyperaemic coronary flow by computing the contrast medium transport time under resting conditions, using frame counting, while the fixed-QFR model uses an empiric flow velocity (0.35 m/s)22. In our study, a further analysis demonstrated a significantly lower AUC for the fixed-QFR model compared with the contrast-QFR model (AUC 0.84 [0.77-0.91] vs 0.88 [0.82-0.93], respectively; p=0.0027 for comparison of AUC) (Supplementary Figure 5). This finding is consistent with previous reports outwith the context of SAS20,22, supporting the value of incorporating patient-specific flow characteristics for QFR calculation even in the presence of SAS. However, whether changes in resting flow after removal of aortic stenosis cause a significant change in the diagnostic performance of QFR deserves further investigation.
Finally, interestingly we noted that the significant impact of AVA on the diagnostic performance of QFR was not found in terms of mean aortic gradient (Figure 4). Compared to AVA, aortic pressure gradient depends more on left ventricular systolic function. In advanced aortic stenosis, a certain degree of ventricular dysfunction is expected, which in some cases can limit the accuracy of mean pressure gradient to reflect the aortic stenosis severity accurately. Low-flow, low-gradient aortic stenosis with reduced ejection fraction can be found in this setting. One can argue that a major degree of microvascular dysfunction could also be expected in this subset. In other words, some patients with a mean pressure gradient <50 mmHg may have reduced left ventricular ejection fraction (LVEF) as a consequence of advanced aortic stenosis (Supplementary Figure 6), which may involve more profound microvascular abnormalities that affect the diagnostic performance of QFR in this subgroup of patients.
QUANTITATIVE FLOW RATIO VERSUS ANGIOGRAPHY
In patients with a primary indication for TAVI or surgical aortic valve replacement, the most recent ESC guidelines recommend coronary revascularisation according to angiographic stenosis severity2. However, in the FFR era this recommendation is challenged by the well documented inaccuracy of angiography in determining the functional relevance of coronary stenoses16, even in the presence of SAS23. In our study, we found a clear superiority of QFR over angiography (AUC 0.88 vs 0.74, p=0.0002) in identifying functionally significant stenoses. Of note, we compared QFR with %DS derived from 3D-QCA, an angiography method that has demonstrated higher accuracy than conventional visual or two-dimensional parameters used in everyday practice24.
In summary, we found a good diagnostic yield of QFR in assessing the functional relevance of coronary stenoses in SAS patients awaiting TAVI, particularly a high negative predictive value (NPV) for the safe deferral of revascularisation. These results make QFR a promising technique in this population since it does not depend on hyperaemic drugs or additional coronary instrumentation. However, large-scale prospective studies are needed to confirm the findings of our study, as well as clinical studies evaluating outcomes when physiology is used to guide revascularisation in this population.
Study limitations
Our study has several limitations. 1) Due to the retrospective nature of the study, the index angiography studies were not performed with a view to analysing QFR. This angiography-based method depends highly on the image quality, precluding QFR calculation or limiting its accuracy if angiography image quality is not suitable. To minimise the effect of angiogram quality on QFR accuracy, we enforced angiographic inclusion criteria following the recommendations of previous QFR studies. It remains plausible that “QFR-aware” angiograms obtained prospectively would increase even further the diagnostic yield of the technique. 2) Given the retrospective nature of the study, and the fact that FFR was performed at the operator’s discretion in most of the participating centres, the results could be subject to selection bias. 3) The route of adenosine administration was not homogeneous in all centres (i.e., intravenous vs intracoronary), mirroring clinical practice across different catheterisation laboratories throughout the world when performing invasive physiological assessment. 4) Agreement between QFR and FFR after TAVI was not evaluated. Despite the increased interest in understanding coronary physiological changes caused by relief of aortic stenosis, data on the effect of TAVI or aortic valve replacement on coronary haemodynamics are still scarce. Furthermore, since transcatheter heart valves can hamper invasive functional assessment of coronary stenoses with catheters and pressure wires, evaluation of the need for coronary revascularisation before TAVI is preferable, and in cases of surgical replacement may facilitate decision making on the best treatment strategy.
Conclusions
Compared with FFR, QFR has a good diagnostic yield and is superior to angiography in assessing the functional relevance of coronary lesions in SAS patients awaiting TAVI, particularly when AVA is ≥0.6 cm2.
|
Impact on daily practice In our study we found that, overall, QFR has a good diagnostic yield in determining the physiological relevance of coronary lesions in patients with concomitant SAS undergoing TAVI. Importantly, unlike FFR, QFR omits the need for vasoactive drugs and pressure wires, avoiding pharmacological adverse effects and additional procedure-related risks in haemodynamically fragile SAS patients. On this basis, QFR has the potential of improving adoption of physiology into the clinical decision workflow in SAS patients with concomitant CAD. However, the clinical evidence regarding the benefit of physiology-guided coronary revascularisation in this population is still scarce and further investigation is warranted. |
Appendix. Study collaborators
Gabriele Venturi, MD; Cardiovascular Division, Department of Medicine, University of Verona, Verona, Italy. Alfredo Nunes -Ferreira-Neto, MD; Quebec Heart and Lung Institute, Laval University, Quebec City, Quebec, Canada. Catherine Liontou, MD, PhD; Hospital Clínico San Carlos, IDISSC and Universidad Complutense de Madrid, Madrid, Spain. Rafael Vera, MD; Hospital Clínico San Carlos, IDISSC and Universidad Complutense de Madrid, Madrid, Spain. Francesco Maria Lauri, MD; Hospital Clínico San Carlos, IDISSC and Universidad Complutense de Madrid, Madrid, Spain. -Fernando Macaya, MD; Hospital Clínico San Carlos, IDISSC and Universidad Complutense de Madrid, Madrid, Spain. Sonoka Goto, MD; Hospital Clínico San Carlos, IDISSC and Universidad Complutense de Madrid, Madrid, Spain. Angela McInerney, MD; Hospital Clínico San Carlos, IDISSC and Universidad Complutense de Madrid, Madrid, Spain. Jihoon Kim, MD; Department of Internal Medicine and Cardiovascular Centre, Heart Vascular Stroke Institute, Samsung Medical Centre, Sungkyunkwan University School of Medicine, Seoul, Republic of Korea. Ki-Hong Choi, MD; Department of Internal Medicine and Cardiovascular Centre, Heart Vascular Stroke Institute, Samsung Medical Centre, Sungkyunkwan University School of Medicine, Seoul, Republic of Korea. Carlos Cortes, MD; CIBERCV, Cardiology Department, ICICORELAB, Hospital Clínico Universitario, Valladolid, Spain. Gabriela Tirado-Conte, MD; Hospital Clínico San Carlos, IDISSC and Universidad Complutense de Madrid, Madrid, Spain. German Armijo, MD; Hospital Clínico San Carlos, IDISSC and Universidad Complutense de Madrid, Madrid, Spain. Pilar Jimenez-Quevedo, MD, PhD; Hospital Clínico San Carlos, IDISSC and Universidad Complutense de Madrid, Madrid, Spain. Nieves Gonzalo, MD, PhD; Hospital Clínico San Carlos, IDISSC and Universidad Complutense de Madrid, Madrid, Spain. Ivan J. Nuñez-Gil, MD, PhD; Hospital Clínico San Carlos, IDISSC and Universidad Complutense de Madrid, Madrid, Spain. Pablo Salinas, MD, PhD; Hospital Clínico San Carlos, IDISSC and Universidad Complutense de Madrid, Madrid, Spain. Antonio Fernandez-Ortiz, MD, PhD; Hospital Clínico San Carlos, IDISSC and Universidad Complutense de Madrid, Madrid, Spain. Carlos Macaya, MD, PhD; Hospital Clínico San Carlos, IDISSC and Universidad Complutense de Madrid, Madrid, Spain.
Funding
The costs of this study were addressed by the participating institutions, without third party financial support.
Conflict of interest statement
H. Mejia-Renteria reports consultant fees from Medis medical imaging systems bv and speaker fees from Philips Volcano outside the submitted work. A. Kalra reports consulting for Medtronic and Philips. F. Macaya received a grant from Fundación Interhospitalaria Investigación Cardiovascular. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

