
Abstract
Aims: We sought to assess eventual changes in iFR measurements in patients with aortic stenosis (AS) before and after TAVI in coronary lesions with different degrees of angiographic severity.
Methods and results: The functional relevance of 145 coronary lesions was assessed by online iFR and FFR measurement in 66 patients with severe AS before and after TAVI, during the same procedure. The iFR-FFR classification agreement was calculated for pre-TAVI and post-TAVI measurements. Mean iFR values remained identical before and after TAVI, irrespective of the angiographic severity of the coronary stenosis (0.89±0.12 vs. 0.89±0.12, p=0.66). However, individual iFR values varied widely after TAVI and the 0.89 iFR threshold was crossed by 15% of the investigated coronary lesions. Higher iFR variation was related to a higher transaortic gradient drop after valve intervention. The diagnostic accuracy of iFR in predicting an FFR ≤0.8 was poor (65%) in lesions with severe obstructions, and tended to increase post TAVI.
Conclusions: Although overall values did not change after TAVI, iFR presented significant and mostly erratic individual variations after valve replacement. Delta iFR was influenced by the extent of the transaortic gradient drop induced by TAVI. Therefore, caution is advisable in the interpretation of iFR in the presence of AS.
Abbreviations
AS: aortic stenosis
AUC: area under the curve
CAD: coronary artery disease
CFR: coronary flow reserve
DS: diameter stenosis
FFR: fractional flow reserve
iFR: instantaneous wave-free ratio
QCA: quantitative coronary analysis
TAVI: transcatheter aortic valve implantation
TTE: transthoracic echocardiography
Introduction
The optimal strategy to assess the functional severity of concomitant coronary artery disease (CAD) in aortic stenosis (AS) is difficult to define1. Moreover, coronary flow reserve (CFR) is attenuated in patients with AS, even in the absence of coronary obstructions2.
Fractional flow reserve (FFR) might have some limitations in severe AS, mainly because of the impaired maximal hyperaemia achievable due to the microvascular dysfunction frequently observed in AS2-5. Recently, we demonstrated that, although overall FFR values do not change before and immediately after TAVI, positive and borderline FFR values significantly decrease after TAVI6.
The instantaneous wave-free ratio (iFR) is a hyperaemia-free index calculated at rest without the need for pharmacologic vasodilation7. iFR has been proposed as an alternative means for assessing CAD in AS without the need for high doses of vasodilators1 and, as such, it may be preferred in patients with severe AS. However, iFR has never been validated before in AS patients.
In the present analysis, we formulated the hypothesis that iFR is influenced by the presence of severe AS. The changes in pressure load and coronary flow that are going to occur immediately after TAVI are likely to alter the resting conditions investigated by iFR. Indeed, it has been demonstrated that CFR, closely linked to iFR8, increases significantly after TAVI9. Therefore, the results of physiological assessment by means of iFR may vary significantly after aortic valve replacement, especially in coronary lesions with borderline results at the pressure wire interrogation.
Methods
The study protocol has been described previously6. Briefly, in this prospective, observational study, iFR and FFR measurements were attempted in the three major epicardial coronary arteries of patients with AS and associated CAD before and immediately after TAVI, maintaining similar haemodynamic conditions. We assessed the eventual changes in iFR measurements before and after aortic valve replacement in different subgroups of coronary lesions. In particular, we investigated those coronary lesions in which the iFR value crossed the 0.89 cut-off value after TAVI, changing the indication to myocardial revascularisation.
The study was approved by the ethical review board of the University of Verona (ID CESC 2015-498), and all patients eligible for the protocol provided written consent.
Fifty-four out of the 66 patients reported here belong to a series previously reported6.
PATIENT SELECTION
Patients included in the study had severe, symptomatic AS defined according to current ESC Guidelines10, at least one coronary artery stenosis, and a clinical indication to elective TAVI as jointly evaluated by the Heart Team. Inclusion and exclusion criteria were the same as reported previously6.
Severe AS was confirmed by transthoracic echocardiography (TTE) as recommended. Aortic gradients, valvular areas and left ventricular function were measured before and after valve replacement using TTE. All TAVI procedures, including haemodynamic, iFR and FFR measurements, were performed by the percutaneous transfemoral approach under local anaesthesia and only mild conscious sedation. None of the patients included in the analysis required intravenous pressure support.
The choice of the aortic valve prosthesis was left to the operator’s discretion. The Medtronic CoreValve® Evolut™ R bioprosthesis (Medtronic, Minneapolis, MN, USA) or Edwards SAPIEN 3 bioprosthesis (Edwards Lifesciences, Irvine, CA, USA) was used in this study.
CORONARY ANGIOGRAPHY AND QUANTITATIVE CORONARY ANALYSIS
Coronary angiography was performed by a standard percutaneous femoral approach with 6 Fr guiding catheters using the same vascular access predetermined for the valve implantation11. The severity of the CAD was graded by QCA performed off-line by expert operators blinded to the FFR and iFR results using the software package CAAS-II QCA (Pie Medical Imaging, Maastricht, the Netherlands) in a previously validated core laboratory (NBR, Verona, Italy)12.
Arteries showing minimal angiographic lesions with a percent diameter stenosis (%DS) ≤30% were considered angiographically unobstructed; those with a %DS ≥30 ≤50 were classified as having intermediate lesions, and those with %DS >50% as having significant lesions.
INTRACORONARY PRESSURE MEASUREMENTS
A pressure monitoring guidewire (PrimeWire®; Volcano Corporation, Rancho Cordova, CA, USA) was advanced distally to the coronary artery stenosis after normalisation. Hyperaemia was obtained after administration of an intracoronary bolus of a high dose (150 to 250 mg) of adenosine as previously indicated by other authors, who reported equivalent diagnostic capabilities compared to i.v. infusion9,13,14. Nitroglycerine was not administered, given the presence of severe AS6,9. An FFR value ≤0.80 was considered pathologic according to current recommendations14. iFR was measured online before FFR, and using the Volcano iFR computational algorithm. An iFR cut-off value of 0.89 was considered equivalent to the 0.80 FFR value for the determination of ischaemia-provoking stenosis15.
STATISTICAL ANALYSIS
Continuous variables are presented as mean and standard deviation (SD) whereas categorical variables are presented as frequencies (percentages).
Comparison of variables before and after TAVI was performed using a repeated measures mixed model, with vessel nested within patient. The TAVI effect was considered as a binary variable (before vs. after TAVI). Linear regression analysis was used to test the association of delta iFR between pre and post TAVI with other variables.
Sensitivity, specificity and area under the curve (AUC) were defined from the calculated receiver operating characteristic (ROC) curve. The diagnostic accuracy of iFR was defined as the proportion of correctly classified lesions (against FFR) among all coronary obstructions.
A p-value <0.05 was considered statistically significant. All statistical analyses were performed using Stata/SE 14.0 (StataCorp LP, College Station, TX, USA).
Results
PATIENT POPULATION
Between January 2015 and February 2017, 66 patients with severe and symptomatic AS with concomitant CAD underwent TAVI and concluded all the study protocol steps, that included the online iFR and FFR measurements before and after TAVI on 145 coronary lesions.
Twenty-one patients received a CoreValve Evolut R valve, and 45 patients received an Edwards SAPIEN 3 valve. Baseline and angiographic characteristics of the overall patient cohort are shown in Table 1.
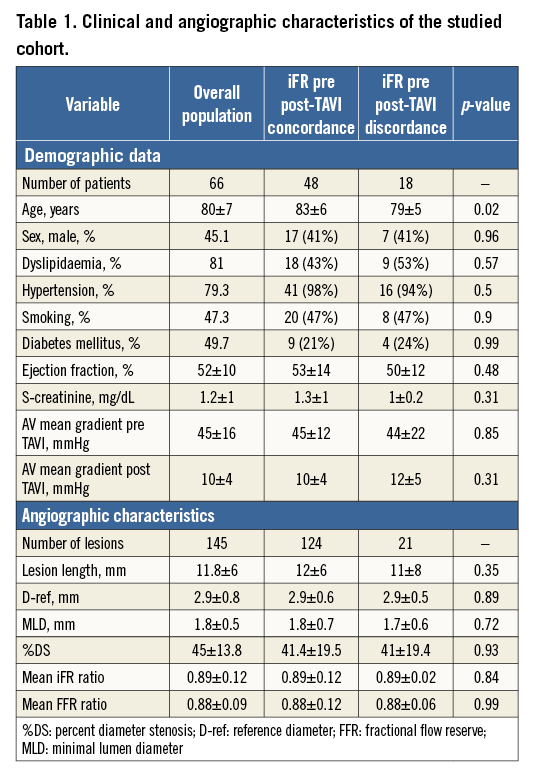
Patients had predominantly angiographically intermediate stenosis, with 80% of lesions showing between 30 and 70 %DS following a normal distribution. Mean percentage diameter stenosis was 45±13.8% (Figure 1). Mean pre-TAVI FFR and iFR were 0.88±0.09 and 0.89±0.12, respectively. The proportion of stenosis with post-TAVI FFR values lower than 0.80, 0.75, 0.60, and 0.50 was 22.6%, 14.6%, 5.8%, and 1.4%, respectively.
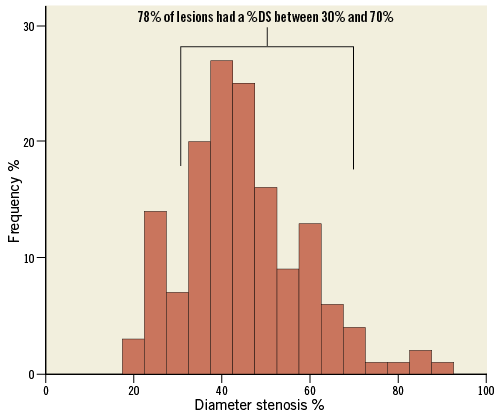
Figure 1. The majority of lesions were classified as angiographic intermediate, with mean %DS 45%±13.8 and 78% of %DS values between 30% and 70%.
EFFECT OF TAVI ON FUNCTIONAL ASSESSMENT OF CORONARY LESIONS
During the whole procedure all patients remained awake, and their haemodynamic parameters did not change before and after TAVI (Table 2).
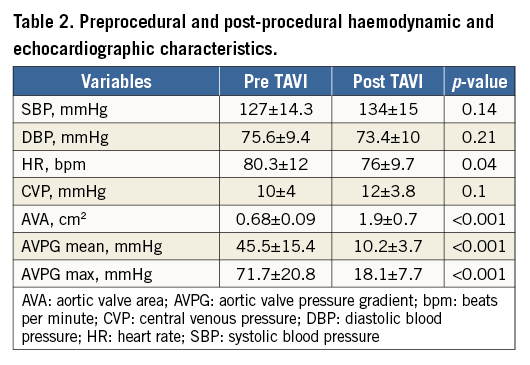
TAVI effect had no significant interaction with the overall iFR measurements (z=0.44, p=0.66). In fact, overall iFR values did not change after TAVI compared with baseline (0.89±0.12 vs. 0.89±0.11). Even including the baseline iFR group in the model as a binary variable (positive if the ratio was less or equal to 0.89 and negative if greater than 0.89), its interaction with TAVI effect was not statistically significant (z=0.05, p=0.82), indicating no significantly different trend in the average iFR values between the two groups.
Positive iFR values (≤0.89) at baseline were found in 58 out of 145 (40%) lesions. The average iFR measurements in this subgroup were comparable after TAVI (0.77±0.13 vs. 0.79±0.11). Similarly, no significant variations were found before and after TAVI in the subgroup of coronary lesions with negative iFR value (0.96±0.03 vs. 0.95±0.05).
No significant differences were found comparing the clinical and angiographic characteristics of the two subgroups. A trend towards higher serum creatinine was observed among patients with negative baseline iFR (1.42±1 vs. 1.1±0.33, p=0.06).
When the coronary vessel was added to the model (LAD versus non-LAD), no significant interaction with TAVI effect was evident (χ²=4.64, p=0.1), indicating a similar trend between the two groups. Mean iFR values in LAD varied from 0.84±0.11 at baseline to 0.85±0.11 after TAVI. In non-LAD coronary arteries, the iFR measurements were 0.93±0.1 before TAVI and 0.93±0.13 after TAVI.
EFFECT OF TAVI ON CORONARY LESIONS STRATIFIED BY QCA
When %DS was included in the model as a categorical variable, its interaction with TAVI had no statistical significance (χ²=1.84, p=0.39), indicating a similar trend between coronary lesions with %DS ≥50 and those with %DS <50. In fact, the average iFR measurements were comparable before and after TAVI both in coronary obstructions with %DS ≥50 (0.79±0.11 vs. 0.78±0.11) and in those with %DS <50 (0.94±0.15 vs. 0.93±0.1).
iFR DIAGNOSTIC PERFORMANCE AND iFR-FFR CLASSIFICATION AGREEMENT
The iFR diagnostic performance in predicting a positive FFR was high before and after TAVI (AUCpreTAVI=0.90, CI: 0.84-0.96 vs. AUCpostTAVI=0.93, CI: 0.88-0.97, p=0.71) (Figure 2). Using the 0.89 cut-off, the diagnostic accuracy of iFR was 76.5% before TAVI and 82.3% at post-TAVI assessment (p=0.27) (Figure 3).
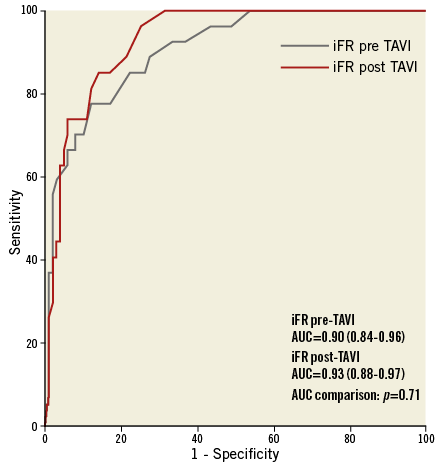
Figure 2. ROC curve comparison of iFR measurements before and after TAVI in predicting a post-TAVI positive FFR (≤0.8).
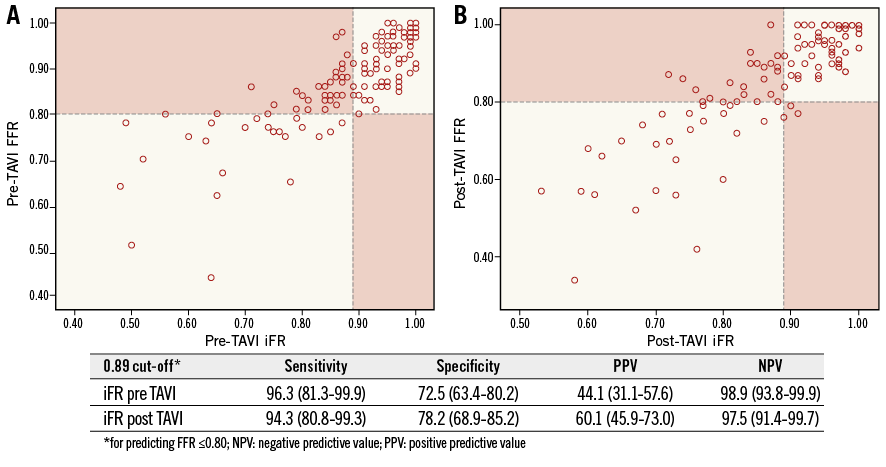
Figure 3. iFR-FFR classification agreement. A) Before TAVI. B) After TAVI. Dashed lines indicate the 0.80 FFR and 0.89 iFR cut-offs. The red blocks indicate the iFR-FFR disagreement zones.
In coronary lesions with %DS <50, the iFR-FFR agreement was elevated and similar before and after TAVI (83.15 vs. 84.8%, p=0.91), whereas in those lesions with significant angiographic obstructions (%DS ≥50), the iFR-FFR agreement was poorer and tended to increase after TAVI (65.3 vs. 78.7%, p=0.08).
Similarly, the iFR-FFR agreement was found to be poor in coronary lesions with baseline positive iFR values, both before TAVI and after TAVI (43.1% vs. 57.9%, p=0.15). On the other hand, in those lesions with baseline negative functional evaluation (iFR >0.89), the iFR-FFR agreement was significantly higher (p<0.0001) compared with functionally significant obstructions (iFR ≤0.89) whether before TAVI or after TAVI (98% vs. 90.1%, p=0.06).
INDIVIDUAL iFR CHANGES BEFORE AND AFTER TAVI
Although the mean iFR value did not change, a wide variation in the individual iFR measurements was observed before and after TAVI (Figure 4A).
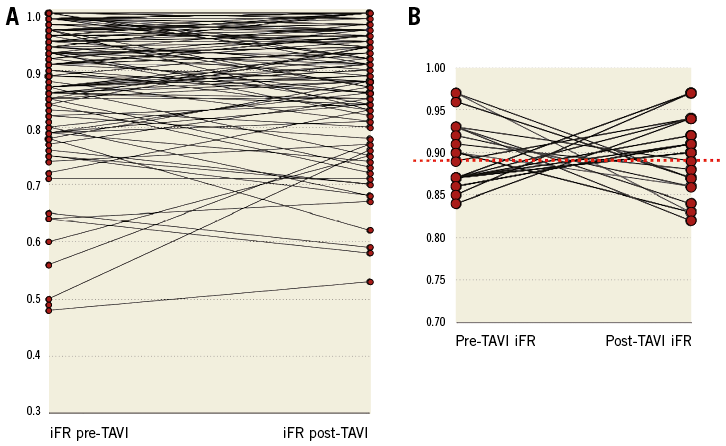
Figure 4. Individual iFR variations before and after TAVI. A) Overall individual iFR values in patients with AS before and after TAVI. B) Subgroup of coronary lesions that crossed through the 0.89 iFR threshold after TAVI. Red dashed line indicates the 0.89 iFR threshold.
Twenty-one out of 145 (14.5%) overall coronary lesions in 18 out of 66 (27.3%) patients crossed the 0.89 threshold after TAVI (Figure 4B). Negative baseline iFR values (>0.89) shifted below the 0.89 threshold in 10 out of 87 (11.5%) coronary lesions after TAVI. Baseline positive iFR values (≤0.89) shifted above the 0.89 threshold in 11 out of 58 (18.9%) coronary lesions. Of note, all these cases showed intermediate lesions at QCA (%DS range, 37-70) and similar lesion length (10.9±7.2 mm) compared with that of coronary obstructions that did not cross the iFR cut-off (12.3±5.7 mm, p=0.53).
Of note, the FFR crossed the 0.8 threshold in only four out of 21 cases in this subgroup of lesions. Overall, FFR values crossed the 0.80 cut-off in 12 (8.3%) lesions and 10 (15%) patients.
No significant differences were observed between the clinical characteristics of patients with coronary lesions that crossed the 0.89 iFR threshold after TAVI compared with the rest of the study cohort. The only exception was the younger age of the group of patients who presented discordance in the iFR measurements pre and post TAVI (79±5 vs. 83±6, p=0.02) (Table 1).
DETERMINANTS OF INDIVIDUAL iFR VARIATION AFTER TAVI
The average delta in iFR measurements before and after TAVI was –0.004±0.063 (Figure 5).
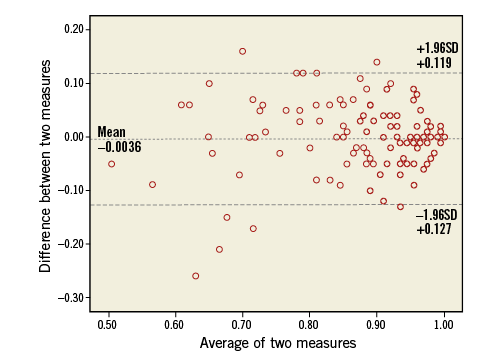
Figure 5. Bland-Altman plot of iFR pre- and post-TAVI measurements.
Higher iFR variation before and after TAVI was associated with higher baseline mean aortic gradient (r=0.274, p=0.009) and with a higher transaortic gradient drop after valve replacement (r=0.494, p<0.0001).
Conversely, the delta iFR pre and post TAVI was not significantly influenced by other baseline patient characteristics such as age (r=0.57, p=0.52), LV ejection fraction (r=0.078, p=0.439), interventricular septum wall thickness (r=0.184, p=0.207), diabetes (r=0.045, p=0.619), hypertension (r=0.029, p=0.799), and baseline renal function (r=0.06, p=0.529). Similarly, coronary lesion angiographic features such as %DS (r=0.035, p=0.69), lesion length (r=0.01, p=0.91) and reference vessel diameter (r=0.026, p=0.78) did not influence the iFR variation pre and post TAVI.
Similarly, the iFR values pre and post TAVI were not influenced by procedural variables such as heart rate (r=0.056, p=0.31) and blood pressure variations (r=0.09, p=0.23) after TAVI.
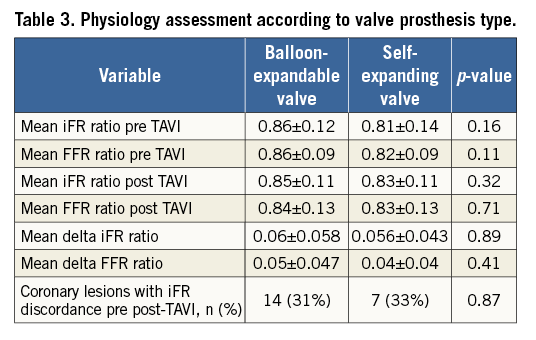
Likewise, no significant difference in functional measurements was detected between patients treated with balloon-expandable valves and those treated with self-expanding valves (Table 3).
Discussion
This study reports the very first observation of real-time iFR measurements in patients with severe AS undergoing TAVI. The main findings of the study are the following:
1) Overall iFR values remained stable before and after TAVI, along a broad range of coronary stenosis severity. However, individual iFR measurements showed a wide and erratic variation after TAVI in up to 15% of coronary lesions that crossed the 0.89 threshold after TAVI, potentially changing the indication for treatment between a pre- and a post-TAVI assessment in 27% of patients.
2) iFR yielded good diagnostic accuracy in predicting positive FFR. Indeed, the 0.89 cut-off demonstrated a high negative predictive value (NPV), so that significant lesions (defined as FFR ≤0.8) would not be missed.
3) The iFR-FFR classification agreement is generally poorer in coronary obstructions with more severe angiographic and functional characteristics.
4) Delta iFR appeared to be influenced by the transaortic gradient drop induced by valve replacement but not by other patient or lesion characteristics.
Functional assessment with FFR in TAVI patients is feasible and safe6,9,16. However, no validation of the FFR ischaemic cut-off in AS is available17, and some concerns are perceived among interventionalists regarding the use of vasodilators in this setting18.
Several factors might limit the achievement of maximal hyperaemia in patients with AS and therefore influence the FFR measurements. It has been proposed that some of the effects of AS on coronary physiology are immediately reversible after relieving the valve pressure gradient19, potentially increasing the reliability of the post-TAVI FFR measurements1.
iFR has demonstrated a high diagnostic agreement with FFR in stable CAD patients20-22, and recent large randomised trials demonstrated the non-inferiority of iFR-guided revascularisation compared to FFR-guided revascularisation23,24. In the specific setting of AS, iFR might facilitate a wider adoption of functional assessment of coronary lesions by obviating the need for high doses of vasodilators.
Although the average iFR measurements were identical before and after aortic valve replacement, a case-by-case analysis disclosed some erratic individual variations of iFR values between the pre-and post-TAVI measurements (Figure 4). Indeed, using the current 0.89 iFR cut-off, the indications to treat a certain stenosis would be changed in 14.5% of lesions, and in 27.3% of patients after TAVI.
Recent data from the DEFINE-FLAIR and SWEDEHEART trials showed that the number of functionally significant coronary stenoses was lower in the iFR group than in the FFR group23,24. Interestingly, we observed an opposite trend in patients with severe AS25. The resting conditions in AS are in fact very different compared with CAD patients without aortic valve disease. The presence of baseline microvascular dilatation in AS has the function to compensate for the decreased perfusion pressure related to the valve obstruction9. At the same time, the microvascular resistances increased in severe AS because of endothelial dysfunction and extracellular compression forces3. The combination of these conditions leads to an increased resting flow, CFR exhaustion and to consequently lower iFR values.
The lower iFR values observed in AS compared with the average FFR values might reflect the lower CFR in AS8. On the other hand, the impaired hyperaemic response elicited by adenosine in AS may limit the achievement of maximal hyperaemic flow, resulting in a lower hyperaemic gradient and therefore in a higher FFR value. These concomitant conditions may be responsible for the different behaviour of iFR and FFR in AS compared with stable CAD patients.
These observations are supported by the recent evidence of a lower difference on average between the two indices calculated by Bland-Altman analysis and of a lower iFR cut-off for matching an FFR ≤0.8 in AS compared with CAD (0.83 vs. 0.89)25.
Interestingly, a lower rate of FFR variations across the cut-off was observed compared with iFR (8.3% vs. 14.5% coronary lesions) in this study. This phenomenon could possibly be explained by the fact that the hyperaemic response elicited by adenosine is not instantly and completely restored by TAVI. As a consequence, FFR could be less likely to be able to detect significant variations of pressure gradient across a given stenosis compared with iFR immediately post TAVI.
Patients with more severe aortic valve gradients and higher gradient drop after TAVI showed a wider variation of iFR values. The change in the pressure load that occurs after TAVI reflects the increase in LV driving forces and the consequent increase in coronary flow. The close correlation of iFR with coronary flow measurements might explain the significant variations of iFR observed after TAVI.
Neither patient characteristics nor angiographic features were associated with delta iFR values. As a consequence, individual iFR changes after TAVI resulted in being unpredictable in some coronary lesions within a wide spectrum of angiographic stenosis and functional severity. Nevertheless, the 0.89 threshold yielded an elevated NPV (98%) both at pre- and post-TAVI investigation. This fact is reassuring concerning the possibility of deferring intervention in intermediate coronary lesions without missing ischaemic ones with acceptable accuracy. On the other hand, the poor positive predictive value (PPV) limits the capability of iFR to detect ischaemia-provoking stenosis in AS before TAVI.
Limitations
The main limitation of the present study is that direct measurement of coronary flow was not performed. Assuming that iFR correlates highly with CFR, one may hypothesise that the changes observed in the iFR values depend on the coronary flow variations after TAVI. Therefore, the measurement of coronary flow could explain this point better. Further research is needed to understand iFR behaviour in the presence of severe AS.
Furthermore, no validation of FFR in AS is currently available and the conventional ischaemic cut-off for CAD assessment may vary in this setting.
Lastly, the sample size is limited. Nevertheless, this remains the largest report available on the real-time iFR-FFR assessment of coronary lesions before and immediately after aortic valve replacement.
Conclusions
Overall iFR values remained identical before and after AS removal. However, individual iFR measurements presented some unpredictable variations after the aortic valve replacement. This observation, along with the evidence of a correlation between delta iFR and the extent of the transaortic gradient drop after TAVI, confirms the variability of functional indices before and after the aortic valve replacement, and underlines the need for further studies dedicated to understanding iFR and FFR changes in severe AS.
| Impact on daily practice The peculiar setting of coronary microvascular circulation and the reduced coronary flow reserve in patients with severe aortic stenosis (AS) influence the iFR assessment of a bystander coronary stenosis. Our study demonstrated that iFR values crossed the 0.89 threshold in 15% of coronary lesions for 27% of patients after TAVI, potentially changing the indication for treatment. The interpretation of iFR measurement in patients with severe AS requires caution since it might be altered by the distinctive coronary conditions caused by the valve obstruction, and it might change significantly after aortic valve replacement. |
Funding
This study was partially supported by a scholarship grant from Volcano Corporation.
Conflict of interest statement
The authors have no conflicts of interest to declare.

