
“Accept no substitutes!”. Does this slogan belong solely to the domain of the advertiser, or might it apply to medicine beyond generic drug substitutions at the pharmacy? The QASTA study, published in this issue of EuroIntervention1, demands that we address the question in regard to coronary physiology for patients with aortic stenosis (AS) undergoing transcatheter aortic valve implantation (TAVI).
In that study, 138 lesions from 7 centres in 5 countries underwent fractional flow reserve (FFR) assessment as part of the TAVI workup, then post hoc analysis of the angiograms to compute the quantitative flow ratio (QFR). Does QFR provide an acceptable substitute for FFR?
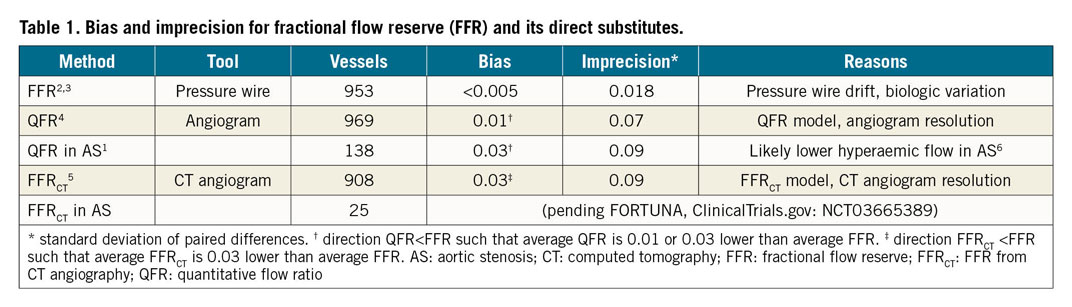
Bias and imprecision
Substitutes for FFR fall into three general categories. Tools such as QFR and other anatomically based physiologic simulations, including FFR from computed tomography (FFRCT), aim to provide the same numeric value as invasive FFR but without the pressure wire (in the case of QFR) or without invasive angiography (in the case of FFRCT). Alternatively, non-hyperaemic pressure ratios avoid vasodilator stress but keep the invasive pressure wire. Finally, non-invasive perfusion imaging uses the related concepts of absolute or relative flow. In this editorial, we concentrate on the first category that one might call “direct” substitutes since the numeric values should be exactly the same.
A direct substitute can suffer from either of two problems. First, its typical value might differ in a systematic way, which is termed “bias” and is computed from a Bland-Altman analysis as the mean difference. Second, the substitute might show great variability for the same conditions, which is termed the “limits of agreement” when computed as the 95% confidence interval (CI) of differences from a Bland-Altman analysis, although it could also reasonably be called “imprecision”.
Our Table 1 summarises these two properties for FFR and its proposed substitutes2,3,4,5, bias (mean difference) and imprecision (standard deviation of differences). For FFR, these values come from studies that repeated the measurement a short time later, as if “substituting” FFR for itself. For QFR and FFRCT, these values come from pooled, lesion-level analyses comparing FFR against its substitute. Bias remains small for all techniques, while the key difference involves imprecision. Table 1 shows that an invasive FFR value of 0.75 should be considered as 0.75±0.02 (95% CI: 0.71 to 0.79), whereas a QFR value of 0.75 should be considered as 0.75±0.07 (95% CI: 0.61 to 0.89). Is this “positive” QFR really positive when viewed alongside its imprecision?
What about aortic stenosis?
With this conceptual background, we can better appreciate the implications of the current study applying QFR in patients with severe AS undergoing TAVI1. The key result is demonstrated in their Supplementary Figure 2 showing a larger bias (QFR
An indirect analysis that could have been performed with the acquired data would have been a “dose/response” relationship. Presumably, more severe AS should lead to further reductions in hyperaemic flow and therefore a growing divergence between QFR and FFR. Unfortunately, Figure 4 of the manuscript only provides accuracy that does not account for potential changes in the FFR distribution with worsening AS. Furthermore, if the decrease in ejection fraction seen in their Supplementary Figure 6 indicates myocardial injury from AS, then maximum hyperaemic flow should fall in parallel, and QFR should be lower than FFR across this spectrum. Sadly, summary figures of accuracy without scatter plots or multivariable models preclude such insights that could have been gleaned from the existing data.
Ultimately, the modest reference vessel diameter (25% of vessels smaller than 2.5 mm with median diameter 2.8 mm) and mild physiologic severity (median FFR 0.84 with 75% of the cohort 0.77 or greater) indicate lesions with lower risk located in distal branches supplying less important amounts of myocardium. While we await ongoing randomised trials of percutaneous coronary intervention (PCI) in patients with severe AS (isrctn.com ISRCTN75836930 and clinicaltrials.gov NCT03058627 and NCT03360591), if we are ever to see an advantage from PCI in this population then we should focus on more severe and more proximal lesions.
The observation that FFR can fall in some cases after TAVI due to an improvement in hyperaemic flow, in both the short and long term6, raises the question of whether pre-TAVI QFR might agree better with post-TAVI FFR. While not addressed in the current study1, we encourage investigators to test this hypothesis in the future. However, the results would only be expected to revert the bias and imprecision of QFR back to values seen in general patients, not outperform pre-TAVI invasive FFR.
Will ongoing trials of FFR substitutes help?
On the basis of large, international, randomised trials with long-term follow-up7,8, we believe that FFR has earned its place as the invasive reference standard. Emerging, albeit observational, data applying FFR during TAVI workup9 suggest that there is no need to proceed any differently with severe AS pending the studies listed earlier. We are aware of three ongoing randomised trials using QFR, two comparing it against angiography (NCT03656848 and NCT03977129) and the other substituting it for invasive FFR (NCT03729739). One of these trials (NCT03977129) is enrolling subjects undergoing on-pump aortic or mitral valve surgery with a target vessel suitable for concurrent bypass grafting. The other trials are enrolling typical, stable lesions being considered for PCI.
What can we expect to learn from these studies? Each vessel can be grouped into one of four categories.
– QFR and FFR abnormal: randomisation accomplishes nothing since these vessels will be treated regardless.
– QFR and FFR normal: again, randomisation leads to the same medical therapy.
– QFR abnormal but FFR normal: conceptually a repeat of prior randomised trials7 but with smaller sample size.
– QFR normal but FFR abnormal: conceptually a repeat of prior randomised trials8 but with smaller sample size.
Therefore, the ongoing trials mix three groups (same treatment, “FAME 1”, and “FAME 2”) but with smaller sample sizes and without the clarity of each category analysed separately. While it might be reasonable to worry that comorbidities in older patients with severe AS undergoing TAVI might mute or negate the benefits of FFR-guided PCI versus medical therapy, the correct method to answer this question is a dedicated trial, as for patients without severe AS8, not a confusing mixture of “three trials in one”.
Substitutes
In summary, substituting QFR for FFR – both in severe AS and in general – erodes the potential benefit of physiology-guided PCI because of incorrect therapy in a substantial number of patients. The term “substantial” is, of course, subjective, and each interventional cardiologist must make up his or her mind as to how much reduction in accuracy remains acceptable. Unfortunately, in our opinion, ongoing trials will offer reduced clarity due to improper designs. While we are physicians and not advertising “Mad Men”, in our practice we only “accept rare substitutes”!
Conflict of interest statement
N.P. Johnson and P.A.L. Tonino have a patent pending on diagnostic methods for quantifying aortic stenosis and TAVI physiology. N.P. Johnson has received internal funding from the Weatherhead PET Center for Preventing and Reversing Atherosclerosis, has an institutional licensing and consulting agreement with Boston Scientific for the smart minimum FFR algorithm (now commercialised under 510(k) K191008), has a patent pending on algorithms to correct pressure tracings from fluid-filled catheters, and has received significant institutional research support from St. Jude Medical (CONTRAST, NCT02184117) and Philips/Volcano Corporation (DEFINE-FLOW, NCT02328820) for studies using intracoronary pressure and flow sensors.
Supplementary data
To read the full content of this article, please download the PDF.

