
Abstract
Determining the optimal treatment strategy for revascularisation of coronary artery stenosis involves the use of fractional flow reserve (FFR). To improve the low clinical uptake of physiological lesion assessment to guide revascularisation, the instantaneous wave-free period (iFR) was proposed as a simpler alternative to FFR that does not require adenosine administration. iFR is calculated as the ratio of blood pressure distal and proximal to a coronary artery stenosis during the diastolic wave-free period. The wave-free period is a part of the cardiac cycle where generation of new pressure wavefronts does not occur and resting microvascular resistance is relatively minimised. iFR indicates the haemodynamic severity of a stenosis, by assessing the extent to which the epicardial stenosis depletes the microcirculatory, autoregulatory reserve. The introduction of iFR and the potential to assess haemodynamic stenosis severity without the need for administration of potent vasodilators such as adenosine sparked an interesting debate about the fundamentals of human coronary physiology. The outcomes of two randomised clinical trials investigating iFR are pending. These studies are designed to evaluate whether iFR-guided revascularisation is non-inferior to an FFR-guided approach. The purpose of this review article is to discuss the physiological concepts underlying iFR, examine the existing validation studies and discuss the advantages and disadvantages of iFR as compared to FFR.
Abbreviations
CAD: coronary artery disease
CFR: coronary flow velocity reserve
FFR: fractional flow reserve
iFR: instantaneous wave-free ratio
PCI: percutaneous coronary intervention
Introduction
Visual angiographic assessment of whether a stenosis impedes myocardial perfusion is subjective and imprecise1,2. Physiological interrogation by coronary artery pressure or blood flow more accurately reflects the haemodynamic significance of coronary stenoses. In clinical practice, myocardial fractional flow reserve (FFR) determines stenosis significance and guides coronary revascularisation3,4. FFR expresses the maximal flow in the presence of a stenosis compared to the maximal flow in the hypothetical absence of the stenosis5. FFR is determined as the ratio between mean pressure distal to a coronary stenosis and mean pressure measured at the tip of the guiding catheter proximal to the stenosis during hyperaemia. To establish maximal vasodilation, administration of a pharmacological agent with potent vasodilatory properties, typically adenosine, is required. After an extensive experimental validation process, clinical guidelines recommended FFR to guide coronary revascularisation based upon the two FAME trials6,7. Despite these stringent recommendations, adoption of FFR-guided revascularisation is limited in clinical practice and is estimated to range between 5 and 10%8. Several factors could explain the poor adoption of FFR. Firstly, the substantial costs of pressure wires may prevent widespread application of FFR. However, these costs are offset by a clear reduction in costs when PCI can be deferred, as well as fewer hospital readmissions associated with adverse coronary events. Secondly, the technical steps of normalisation of pressure waveforms, appropriate establishment of hyperaemia and assessment of pressure drift must be carried out with precision to avoid errors in FFR assessment9. Thirdly, interventional cardiologists still largely underestimate the advantage of physiology and rely primarily upon angiography to guide revascularisation10. Finally, the establishment of adenosine-mediated hyperaemia is time-consuming, costly, alters systemic haemodynamics11, and causes unpleasant side effects, such as atrioventricular nodal conduction disturbances, chest discomfort, nausea, dyspnoea, dizziness, flushing and headache12,13. For the latter issue, multiple solutions have been proposed, such as using regadenoson14, nicorandil15 or contrast medium16 to induce hyperaemia. Avoiding hyperaemia altogether by using the instantaneous wave-free ratio (iFR) for assessment of haemodynamic stenosis significance is another option (Figure 1)17. iFR is defined as the ratio between distal and proximal coronary pressure during the diastolic “wave-free period” under resting conditions over at least five heartbeats (Figure 2). Further measurement instructions are provided in the Online Appendix.
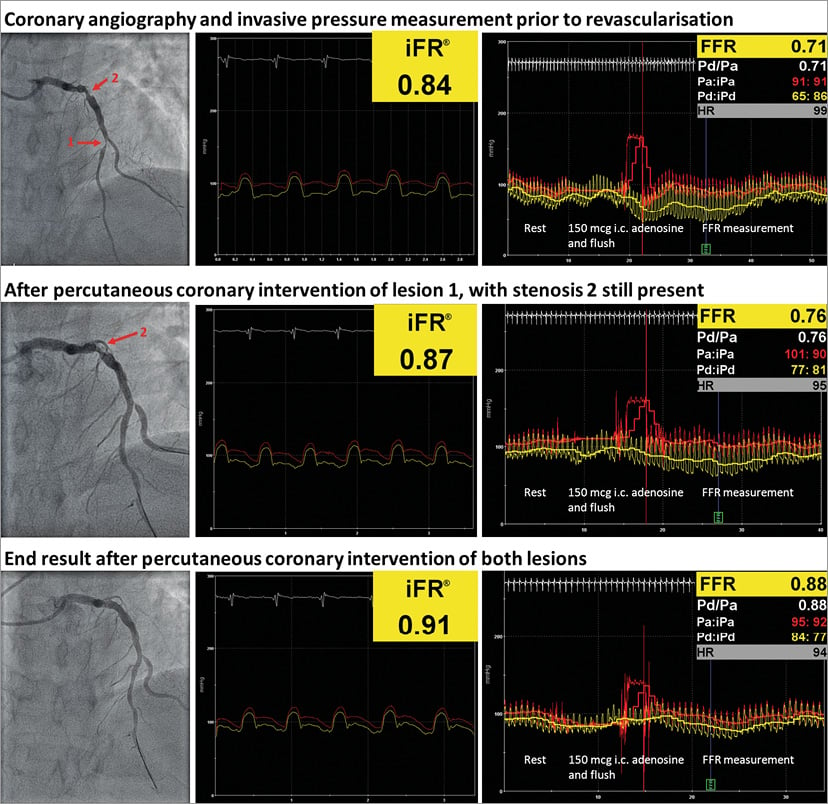
Figure 1. Examples of iFR and FFR measurements. Both iFR and FFR of LAD were significant. After PCI of stenosis 1, both iFR and FFR slightly improved, but were still abnormal. After successful revascularisation of both stenoses, iFR and FFR increased to normal values.
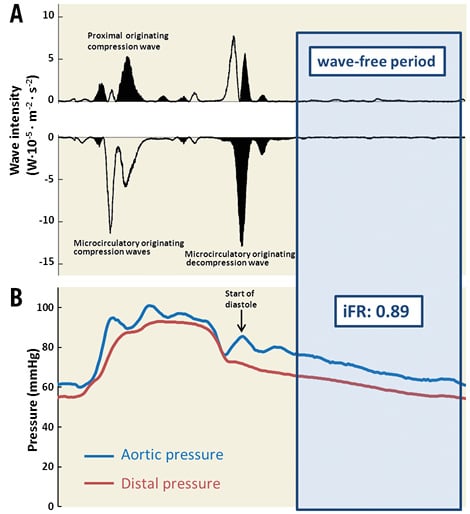
Figure 2. Example of wave-intensity analysis to determine the wave-free period. A) Wave-intensity analysis is performed in a single heartbeat in a 63% stenosis of the first diagonal branch using instantaneous measurements of intracoronary Doppler flow velocity and distal coronary artery pressure. As can be seen, in mid-to-late diastole, no wavefronts occur and the wave-free period is defined as starting 25% of the way into diastole and ending 5 milliseconds before the start of the next systole. B) iFR is calculated as the ratio between the aortic and distal pressure during the diastolic wave-free period.
The purpose of this review is to provide an overview of the theoretical framework of iFR, presently existing evidence and future perspectives. Furthermore, the claimed advantages of iFR compared to FFR and the critique that has been cast on the theoretical concepts behind iFR will be discussed.
HISTORICAL PERSPECTIVE OF BASELINE STENOSIS ASSESSMENT
Andreas Gruentzig was already using a fluid-filled catheter to measure blood pressure proximal and distal to the lesion prior to intervention in the 1970s18. Gruentzig recognised that the transstenotic pressure gradient was an important indicator of lesion severity and of procedural efficacy. Although highly innovative, the measurement via a fluid-filled microcatheter was far from perfect as the microcatheter itself physically obstructs the coronary lumen, causing overestimation of the pressure gradient19. Additionally, it was too bulky to pass tighter and more distal lesions. About ten years later, the development of thin guidewires equipped with a pressure sensor allowed measurement of distal coronary pressure with minimal interference from the physical presence of the instrument across the stenosis. With this pressure wire, Pijls and colleagues first laid out the theoretical and experimental basis for the FFR in 19935. By inducing pharmacological vasodilation, autoregulation is abolished and the relationship between distal perfusion pressure and blood flow is linear. Under these conditions, the ratio between aortic and distal pressure can serve as a surrogate for the relative maximal flow limitation. Unlike other invasive tools to measure stenosis severity such as coronary flow velocity reserve (CFR), slope of the instantaneous hyperaemic diastolic coronary flow velocity-pressure relation and hyperaemic stenosis resistance (HSR), FFR is easy to measure and, together with the compelling outcome data, FFR became the preferred physiological measurement. The resting transstenotic pressure gradient only gained renewed attention more than three decades after Gruentzig had recognised its importance. In 2010, Mamas et al found a strong concordance between the baseline Pd/Pa ratio and FFR20. In 2012, Sen and colleagues employed wave-intensity analysis to find a suitable window during the cardiac cycle where disturbances in microvascular resistance were minimal21. Wave-intensity analysis was carried out by calculating the intensity and direction of coronary wavefronts using the derivatives of intracoronary flow velocity and pressure. Wavefronts are concomitant changes in both pressure and flow velocity wavelets, that can travel in either a forward or backward direction through the coronary artery22. During mid-to-late diastole, Sen et al identified a period where wavefronts did not appear, which they termed the wave-free period (Figure 2)21. The wave-free period starts 25 percent of the way into diastole and ends 5 milliseconds before the start of systole. During the wave-free period, microvascular resistance is stable and a linear relationship exists between distal pressure and flow velocity23. Furthermore, because resting phasic microvascular resistance is relatively minimised during the wave-free period (for that cardiac cycle), both flow velocity and the transstenotic pressure gradient are at their relative peak24. The stable and maximised transstenotic gradient provides seemingly ideal conditions for resting stenosis assessment, and iFR was defined as the ratio between distal and aortic pressure during the wave-free period (Figure 2).
iFR VALIDATION
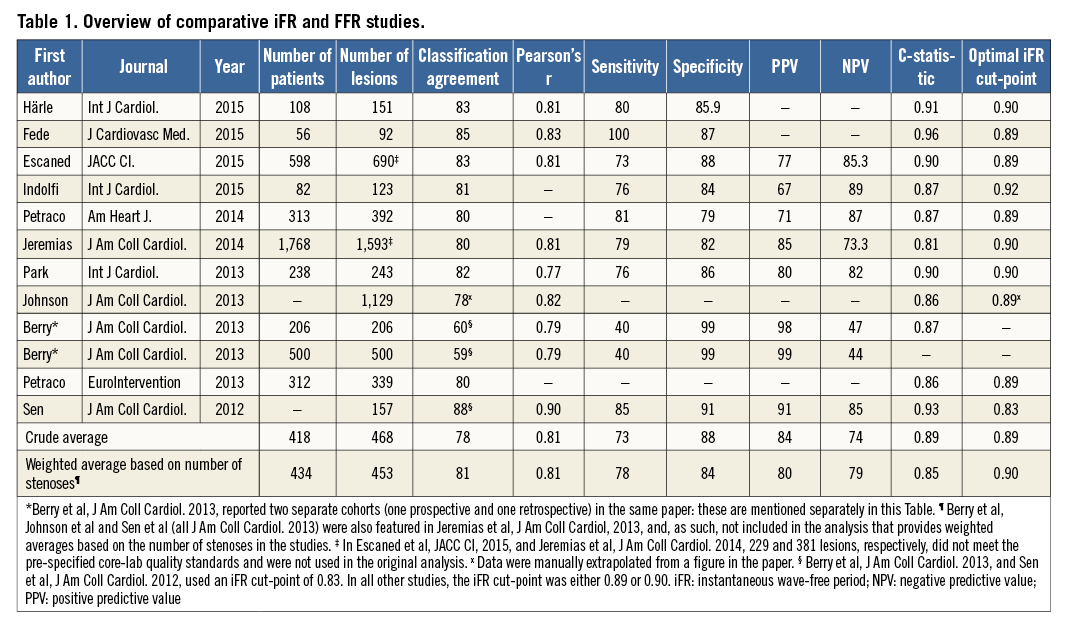
Initial attempts to compare iFR and FFR were undertaken by three different research groups21,25,26. While highly significant correlations between iFR and FFR were consistently found, the strength of the relationship varied between r=0.79 and r=0.90 depending on the distribution of stenoses. The RESOLVE study was conducted to provide a definitive answer with respect to the iFR-FFR relationship27. In this large retrospective study, a correlation coefficient between FFR and iFR of r=0.81 (p<0.001) with a classification agreement of 80.4% was found. In Table 1, an overview of studies comparing iFR and FFR is presented. The interpretation of these studies is difficult, however, since it is unclear whether or not the advantage of obviating hyperaemia is worth the loss in diagnostic accuracy with FFR as reference standard. However, it should be questioned whether direct comparison with FFR should be used to evaluate the clinical applicability of iFR-guided revascularisation. Firstly, FFR is prone to an intrinsic variability of around 4% which a priori precludes perfect classification agreement with iFR28,29. Furthermore, notwithstanding the clinical benefits of FFR-guided revascularisation, FFR should not be considered the gold standard of inducible ischaemia on the myocardial level30. As such, definitive conclusions with respect to the clinical applicability of iFR should not be drawn solely on the basis of its relationship or diagnostic classification agreement with FFR.
Several studies have been conducted using different surrogate measures of myocardial ischaemia. A diagnostically equivalent performance of FFR and iFR was found when compared against non-invasively determined myocardial perfusion impairment as documented by positron emission tomography31 or single positron emission computed tomography32. Similar findings were found using invasive measures of haemodynamic stenosis significance in the CLARIFY17 and JUSTIFY-CFR33 studies. In the CLARIFY study, iFR and FFR showed equivalent diagnostic agreement with HSR determined by simultaneous assessment of Doppler flow velocity and the transstenotic pressure gradient. Earlier work had already shown that the HSR index may be diagnostically superior to FFR34. This provides support for the use of the HSR as reference standard in the CLARIFY study. Of note, in the CLARIFY study, iFR measured with adenosine did not show a better classification agreement with HSR than that of iFR or FFR. In the JUSTIFY-CFR study, FFR and iFR were related to Doppler-defined CFR as the external reference standard. A large burden of evidence suggests that CFR has important prognostic implications, suggesting it may be a reasonable reference standard. In the JUSTIFY-CFR study, iFR had a higher diagnostic classification agreement with CFR than FFR. The superior performance of iFR was predominantly attributed to false positive FFR results in trivial stenoses with a preserved microvascular function and high CFR (Figure 3). In these lesions, strongly increased magnitudes of hyperaemic flow cause turbulence and exacerbate the pressure gradient across the stenosis. Under resting conditions, coronary flow is relatively low, and pressure losses due to flow separation and turbulence at the exit of the throat of such trivial stenoses are minimised. In these cases of high CFR and abnormal FFR, the presence of ischaemia is unlikely and PCI does not appear indicated. Consequently, iFR may provide a more accurate assessment in these cases. This phenomenon is comparable to the elevated pressure gradient found across the aortic valve in high-flow states such as hyperthyroidism, fever or anaemia that normalises when a normal-flow state is restored by reversal of the condition35. In conclusion, while iFR perhaps yields underwhelming results when directly compared with FFR, it is an equally accurate index when compared with external surrogate measures of myocardial ischaemia. Although the surrogate measures of myocardial ischaemia used in the iFR validation studies all have specific drawbacks, the fact that iFR consistently showed either diagnostic equivalence or superiority over FFR suggests that randomised clinical testing is warranted to determine the appropriateness of iFR-guided revascularisation.
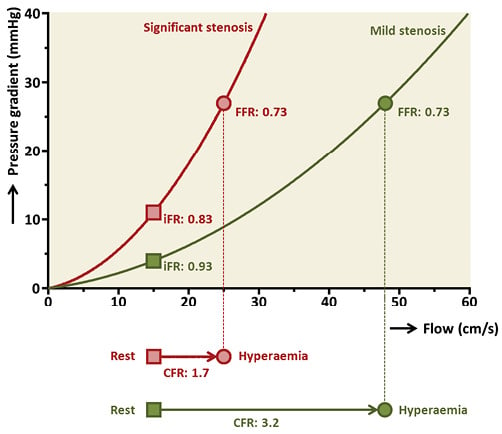
Figure 3. FFR can overestimate stenosis severity in non-flow-limiting lesions. This figure shows how FFR can overestimate stenosis severity as indicated by CFR in non-flow-limiting lesions.
RATIONALE FOR HYPERAEMIA-FREE LESION ASSESSMENT
Ever since the introduction of FFR, the need for hyperaemia to assess lesion severity has been undisputed. This belief was reinforced by the need for hyperaemia to yield diagnostically sufficient accuracy when performing assessment of myocardial perfusion by non-invasive imaging36. iFR challenged the paradigm that hyperaemia is indispensable to assess haemodynamic stenosis significance. Results of the IDEAL study shed light on why coronary hyperaemia may not be necessary to indicate stenosis severity when using coronary pressure specifically24. The IDEAL study showed that, with increasing stenosis severity, the transstenotic pressure gradient concomitantly increases and stable resting coronary flow is maintained by compensatory reduction of microvascular resistance (Figure 4). During hyperaemia, however, the autoregulatory reserve is exhausted and microvascular resistance does not depend on lesion severity. Instead, hyperaemic flow velocity decreases with incremental stenosis severity. The hyperaemic transstenotic pressure gradient is larger than during resting conditions, but also increases with worsening of stenosis severity. In conclusion, for assessment of stenosis severity, a diagnostically sufficient pressure gradient seems to exist under resting conditions. However, when coronary flow or myocardial perfusion is assessed instead of coronary pressure, a hyperaemic agent is unequivocally needed to exhaust the autoregulatory reserve and unmask the presence of myocardial ischaemia. iFR was initially proposed as a method to estimate FFR without the need for hyperaemia, but the results of the IDEAL study considerably changed how the conceptual framework of iFR should be conceived. Based on the IDEAL study, we propose that the theoretical framework of iFR should state that iFR informs on the effect a given epicardial stenosis exerts on the depletion of the autoregulatory capacity that the subtended microcirculation harbours, whereby lower values of iFR correspond to increasing depletion of autoregulatory reserve under resting conditions. This is a key difference from the theoretical framework of the FFR and explains why the correlation coefficient between FFR and iFR is not perfect. Notably, this theoretical framework parallels that of myocardial contrast echocardiography acquired under resting conditions to detect coronary artery stenosis37,38.
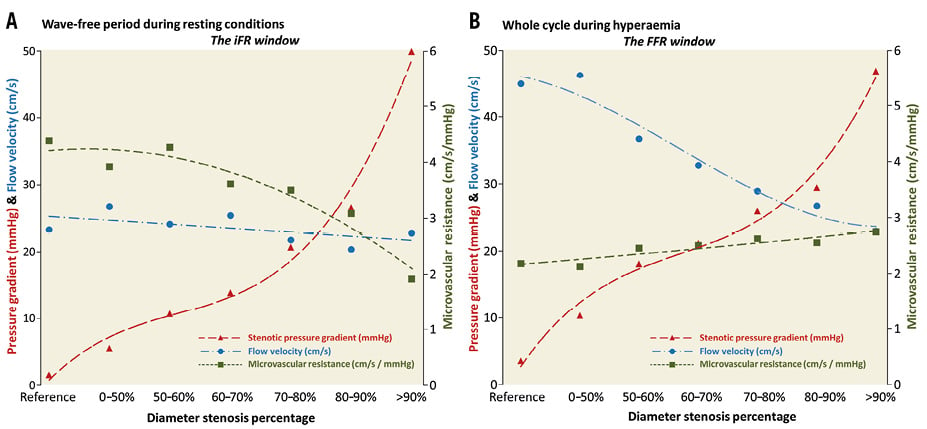
Figure 4. Rationale behind resting pressure assessment. The behaviour of coronary flow velocity, microvascular resistance and the transstenotic pressure gradient according to stenosis severity is shown during the resting wave-free period (iFR window) (A) and during whole cycle under hyperaemic conditions (FFR window) (B). During both the wave-free period and hyperaemia, a transstenotic pressure gradient is detectable that increases with stenosis severity. During the wave-free period under resting conditions, coronary flow velocity is maintained stable by compensatory reduction of microvascular resistance through coronary autoregulation. Data used in this Figure are derived from the IDEAL study24.
iFR CONTROVERSY
Because hyperaemia to assess stenosis significance was broadly presumed to be indispensable, the introduction of iFR elicited scepticism. This paragraph provides a summary of the criticism cast on the foundations of iFR and its use as clinical tool. Criticism with respect to whether the iFR algorithm is required or whether baseline Pd/Pa could also be used is presented in the Online Appendix.
NATURAL HYPERAEMIA DURING THE WAVE-FREE PERIOD?
Findings of the initial ADVISE study showed that microvascular resistance during the wave-free period and hyperaemic whole cardiac cycle was equal in a subset of 39 stenoses with flow velocity and pressure measurements21. This observation hinted that, during the diastolic wave-free period, sufficient vasodilation existed naturally to obviate the need for hyperaemia. Johnson et al later disputed this finding in a larger cohort of 120 patients and noted that resting diastolic resistance was significantly higher than hyperaemic mean cycle resistance26. These discrepant findings are unlikely to be a consequence of methodological inaccuracies in either study. The most logical explanation for the discrepancy is that a different population of stenoses was used. In the IDEAL study, microvascular resistance was stratified according to worsening degrees of stenosis severity24. In unobstructed vessels, microvascular resistance was approximately twofold higher during the resting wave-free period as compared to hyperaemia over the whole cardiac cycle. As stenosis severity increases, hyperaemic whole cycle microvascular resistance remains relatively similar while resting wave-free period microvascular resistance declines. Equipoise between microvascular resistance during the hyperaemic whole cycle and resting wave-free period is reached at approximately 80% diameter stenosis, after which wave-free period microvascular resistance becomes even lower than hyperaemic whole cycle microvascular resistance. Thus, if a population of relatively mild stenoses is selected, microvascular resistance during the wave-free period is higher than during whole cycle hyperaemia. However, if a population of severe stenoses is selected, microvascular resistance during the wave-free period and hyperaemic whole cycle is equal. As such, the assumption that during the wave-free period a phase of “natural hyperaemia” exists is incorrect. However, during the wave-free period microvascular resistance is stable and minimised for that cardiac cycle. In several studies, it was observed that in some stenoses iFR was actually lower than FFR27,39. The IDEAL study demonstrates that this predominantly occurs in severe stenoses where the autoregulatory reserve has been exhausted and no increased flow response occurs in response to hyperaemia24. In these stenoses, adenosine barely lowers resistance or increases the pressure gradient and iFR is lower than FFR because it is calculated during diastole instead of the whole cardiac cycle.
WAVE-FREE PERIOD: FREE OF WAVES?
Westerhof et al critiqued the very foundation of iFR as outlined in the ADVISE study and argued that during the presumed wave-free period waves actually do occur40. By applying wave separation analysis, which is another method to analyse pressure and flow waves, it was reasoned that backward and forward travelling waves in fact do occur during the wave-free period. However, wave intensity calculation is unable to identify these waves because it uses differentiation, whereby the slow changes in diastole are relatively suppressed. Westerhof et al thus claim that pressure and flow waves are present in diastole and the wave-free period is not free of waves. However, the physiological principles of the application of wave separation analysis to diastole have also been criticised41. Tyberg et al postulated that much of this controversy may be explained by a difference in the definition of a wave42. For now, consensus on whether or not the “wave-free period’ is actually free of pressure and flow waves has not been reached. Westerhof et al also criticised the use of the law of Ohm during selected parts of the cardiac cycle and stated that this is physically incorrect and that the law of Ohm can only be applied to mean values of pressure and flow. To prove this, they argued that, if peripheral systemic resistance in the ascending aorta were to be calculated during the diastole specifically, it would be infinite because diastolic flow is negligible, while blood pressure is preserved. Clearly, peripheral resistance is not infinite at any point throughout the cardiac cycle and the value of microvascular resistance can only be correctly derived when averaged over the whole cardiac cycle. Nevertheless, ascending aorta physiology clearly differs from coronary physiology. Westerhof et al note that their criticism is of a fundamental nature and, if clinical results are satisfactory, the use of iFR in clinical practice should not be deterred.
FFR AND iFR IN VARIOUS ANATOMICAL AND PATHOLOGICAL CONDITIONS
FFR has been extensively validated in a variety of different anatomical and pathological conditions. Similar research is steadily being acquired for iFR. In this paragraph, conditions where either iFR or FFR could have an advantage over the other are discussed (non-culprit stenosis assessment in the setting of acute myocardial infarction and aortic valvular stenosis are discussed in the Online Appendix).
DIFFUSE DISEASE AND SERIAL STENOSIS
Diffuse disease and serial stenosis are frequently encountered in clinical practice. By pulling back the pressure wire from the distal end of the vessel towards the ostium of the coronary artery, FFR step-ups can be detected under stable hyperaemic conditions43. The operator can then assess where in the vessel the biggest FFR step-ups occur and look for optimal locations to perform PCI. In the case of serial stenosis, the assessment of FFR is complicated by its dependence on the hyperaemic flow across the lesions. Eliminating one stenosis by PCI causes the total resistance of the vessel to decrease with a subsequent increase in hyperaemic flow across the second stenosis. Because the transstenotic pressure gradient depends on flow velocity (according to the water hammer equations41), the pressure gradient across the remaining stenosis will increase by an unpredictable amount. For this reason, accurate prediction of the contribution of either stenosis towards the overall FFR cannot be made prior to PCI. Resting flow, on the other hand, is maintained at stable conditions by coronary autoregulation. Because iFR uses resting conditions, it appears less prone to interdependence of flow and may lend itself better to predict coronary haemodynamics in PCI of serial stenoses44 (Figure 5). Nijjer et al performed motorised pressure wire pullbacks along diffusely diseased vessels or vessels with serial stenoses to map iFR along its length45. Based on this map, the expected iFR after PCI of one of the stenoses was calculated. The expected iFR before PCI matched the observed iFR after PCI strikingly well (r=0.96, p<0.001). These results suggest that iFR pullback may have an advantage over FFR pullback when guiding PCI in serial stenoses and diffuse disease. This could be of clinical importance in order to minimise stent length and avoid stent-related complications while obtaining a satisfactory physiological result after PCI. An iFR pullback modality is now commercially available (iFR Scout™ pullback technology; Philips Volcano, San Diego, CA, USA), and a system that incorporates the iFR pullback data into the coronary angiogram is in the final stages of development. This pullback module improves the graphical interface of iFR and makes it easier to obtain pullback curves.
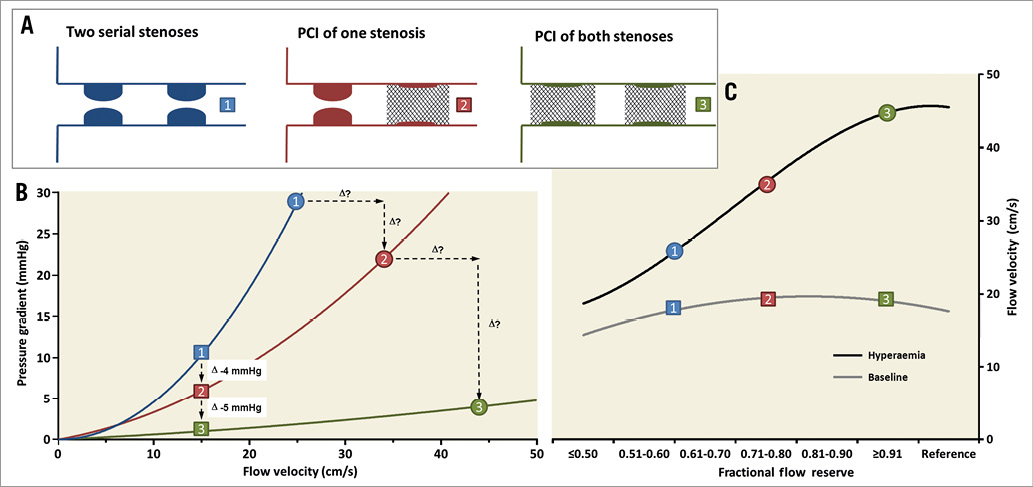
Figure 5. Physiological assessment of serial stenosis. This figure schematically shows how iFR can accurately predict the physiological results after PCI of one stenosis in the presence of serial stenoses by pullback of the pressure wire. Because hyperaemic flow is affected by stenosis cross-talk, prediction of the physiological result is less accurate for FFR. A) Three consecutive steps in the revascularisation of two serial stenoses. B) The classic relationship between flow velocity and pressure gradient as described by Gould et al47. The squares represent resting conditions and the circles represent coronary hyperaemia. C) Resting flow is kept stable across stenosis severities, while hyperaemic flow gradually falls. Because resting flow is similar in scenarios 1 and 2, the pressure gradient across the proximal stenosis does not change when the distal stenosis is treated by PCI. However, hyperaemic flow rises by an unpredictable magnitude if the distal stenosis is treated and the transstenotic gradient across the proximal stenosis is concomitantly increased by an unpredictable amount.
INFLUENCE OF HAEMODYNAMIC FLUCTUATIONS
Fluctuations in heart rate and filling state frequently occur in the catheterisation laboratory, for example due to anxiety, pain or prehydration to prevent contrast-induced nephropathy. Changes in heart rate and loading conditions influence myocardial oxygen demand. Coronary blood flow changes accordingly to match demand by autoregulation46 (Figure 6A). When the hyperaemic state is pharmacologically induced, autoregulation is exhausted and fluctuations in heart rate and filling state will not affect blood flow. As explained earlier, the transstenotic pressure gradient is dependent on the velocity of coronary flow across the lesion. Therefore, haemodynamic variability has been suggested as a source of error in iFR25,26. This reasoning is physiologically sound, but evidence pertaining to the diagnostic impact of haemodynamic variability on iFR is conflicting. The ADVISE study found no correlation between either heart rate, systolic or diastolic pressure and iFR21. This is confirmed in the CLARIFY study, showing that heart rate variability does not appreciably influence the relationship between iFR and either FFR or HSR17. The VERIFY study contravenes these findings and shows that the relative difference between iFR and FFR is significantly correlated with both resting heart rate and blood pressure25. In atrial fibrillation, it is claimed that iFR can still be calculated accurately, and registry studies comparing iFR and FFR did not exclude atrial fibrillation patients. However, it can be envisaged that, if heart rate is not appropriately controlled pharmacologically, myocardial oxygen demand increases. This results in a pseudo-hyperaemic state that is not representative of true resting conditions, which could lead to a falsely decreased iFR. At present, additional evidence is needed to learn whether iFR is truly susceptible to haemodynamic variability.
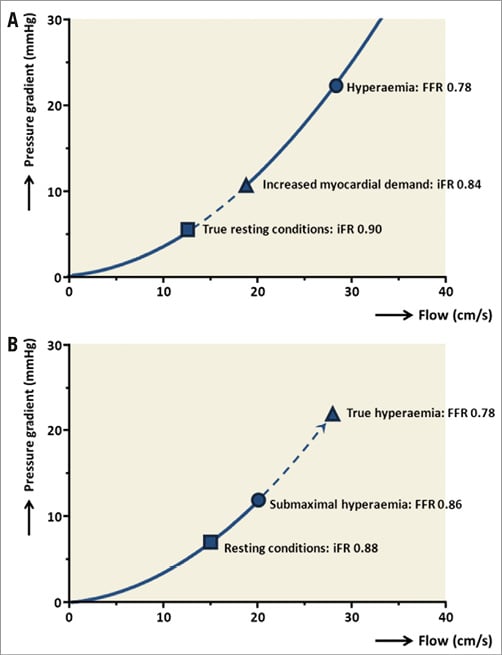
Figure 6. iFR and FFR in specific haemodynamic circumstances. A) When myocardial demand increases, resting flow adapts accordingly through coronary autoregulation. Because the transstenotic pressure gradient is dependent on flow velocity, iFR may be prone to haemodynamic variability. B) If maximal hyperaemia is insufficiently induced, FFR can underestimate stenosis severity.
ATTENUATED RESPONSE TO ADENOSINE
Establishing maximal coronary hyperaemia is crucial to reliable estimation of stenosis severity by FFR and, if hyperaemia is not sufficiently established, FFR may underestimate stenosis severity (Figure 6B). However, microcirculatory dysfunction secondary to hypertrophic or dilated cardiomyopathy, or clinical risk factors such as diabetes mellitus or hypercholesterolaemia, can attenuate maximal hyperaemic coronary flow48. Also, not all patients have a response to adenosine that mimics the hyperaemic coronary response to vigorous exercise49. This resistance to adenosine depends on genetic background50. Furthermore, caffeine and theophylline both competitively antagonise the adenosine receptor and thereby interfere with the actions of adenosine13. As such, insufficient establishment of hyperaemia can occur when theophylline-containing medication or caffeine products are consumed prior to intervention. Therefore, it is advised to discontinue these substances 12 hours prior to FFR measurement13. These examples illustrate that maximal hyperaemia is not always established when measuring FFR. These considerations are not applicable to iFR and this may speak to its advantage.
FUTURE OF iFR
The compiled evidence discussed in this review indicates that iFR is a promising novel tool that can be readily employed to guide coronary revascularisation. Before iFR is used routinely, randomised clinical testing is required and two randomised clinical trials are currently being performed. Both trials are assessing the non-inferiority of iFR- compared to FFR-guided revascularisation of intermediate stenoses with respect to clinical outcomes. Functional Lesion Assessment of Intermediate Stenosis to Guide Revascularisation (DEFINE-FLAIR; ClinicalTrials.gov Identifier: NCT02053038) randomises 2,500 patients 1:1 to either iFR- or FFR-guided revascularisation51. The trial recruits patients from 47 centres in 18 countries across Europe, North America, Africa and Asia. The primary endpoint is a composite of death, myocardial infarction and unplanned revascularisation at one-year follow-up. Results are expected to be available at the beginning of 2017. The Evaluation of iFR vs. FFR in Stable Angina or Acute Coronary Syndrome study (iFR-SWEDEHEART; ClinicalTrials.gov Identifier: NCT02166736) has a similar design to DEFINE-FLAIR but randomises 2,000 patients in Scandinavia52. iFR-SWEDEHEART derives its clinical outcomes from registry data acquired in the Swedish Coronary Angiography and Angioplasty Registry (SCAAR), and results are also expected in 2017. If the DEFINE-FLAIR and iFR-SWEDEHEART trials demonstrate non-inferiority of iFR-guided revascularisation, iFR may receive a similar IA recommendation in clinical guidelines as is currently issued for FFR3,4. Operators will then be faced with selecting the appropriate physiological modality for each individual patient. As discussed throughout this review, evidence suggests that there may be a preference for iFR over FFR in specific situations and vice versa in other situations (Figure 7). Another option is a hybrid FFR and iFR strategy, something which is further discussed in the Online Appendix53.
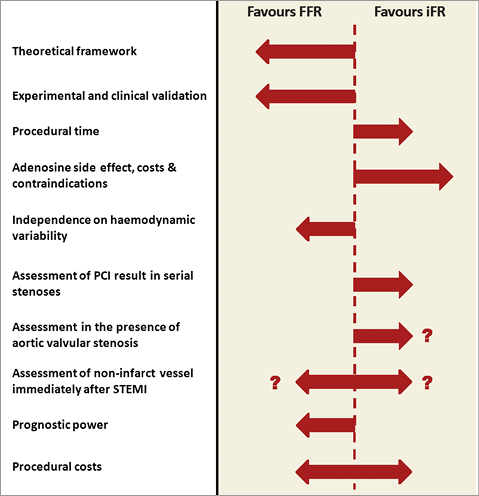
Figure 7. Advantages and disadvantages of iFR compared to FFR. Long arrows indicate that existing evidence strongly favours the modality, while the short arrows indicate some advantages. Question marks (?) indicate that the presumed advantage is based on observed flow changes that were theoretically extended to changes in the pressure ratio’s iFR and FFR.
In patients with clear contraindications to adenosine, such as severely obstructive pulmonary disease, high-grade atrioventricular nodal blocks or profound arterial hypotension, FFR measurement cannot be performed. Because regulatory agencies in both Europe (CE mark) and the USA (Food and Drug Administration) have approved the use of iFR. it can now be used in patients with contraindications to adenosine.
Finally, it should be kept in mind that, while a rigid, dichotomous cut-point of 0.80 or less to indicate revascularisation is used for FFR, the value of FFR represents a continuum where lower values indicate that myocardial perfusion is increasingly compromised5. For instance, an FFR value of 0.83 corresponds to a 17% reduction in coronary blood flow caused by the stenosis. For iFR, a dichotomous cut-point of 0.90 exists that corresponds to the FFR cut-point of 0.8027. However, iFR does not have a similar conceptual framework where values of iFR represent a given loss in coronary flow. Furthermore, lower FFR is directly associated with worse outcomes54. As such, the clinical implications in terms of both reduction in blood flow and prognostic value of specific FFR results are ingrained into the minds of physicians. However, the conceptual framework of iFR should be interpreted differently from that of FFR. iFR relies on the downstream effects of the epicardial stenosis on the microcirculatory autoregulatory reserve, and therefore measured iFR values cannot be tied directly to a given reduction in coronary blood flow. Furthermore, in contrast to FFR, the prognostic implications for specific iFR values have not yet been investigated. Future scientific efforts should be expanded towards further delineation of the conceptual and prognostic framework of iFR to help physicians to understand better the implications of specific iFR values.
| Impact on daily practice iFR is a promising new physiological tool to guide revascularisation of intermediate coronary artery disease that does not rely on pharmacological hyperaemia, but rather on the natural depletion of the autoregulatory reserve owing to the stenosis. FFR and iFR have similar diagnostic accuracy when compared to different surrogate measures of myocardial ischaemia, but have different strengths and weaknesses. The non-inferiority of iFR- compared to FFR-guided revascularisation is being investigated in two clinical trials and the outcomes will decide whether iFR-guided revascularisation can be used in clinical practice. |
Funding
G. de Waard is supported by a Netherlands Heart Institute Fellowship Grant.
Conflict of interest statement
The authors have no conflicts of interest to declare.
SUPPLEMENTARY DATA
Online Appendix. Instantaneous wave-free ratio to guide coronary revascularisation: physiological framework, validation and differences from fractional flow reserve
MEASUREMENT OF iFR
With the exception of hyperaemia, measurement of iFR is largely similar to FFR, and proper normalisation of aortic and distal pressure, administration of nitrates and pressure drift assessment are imperative. iFR should be measured under true coronary resting conditions: i.e., no immediately preceding intracoronary injections of saline, contrast agent or other substances. A specialised iFR algorithm embedded within the iFR console (proprietary to Philips Volcano, San Diego, CA, USA) identifies the wave-free period window. iFR is calculated as the ratio of mean distal to mean aortic pressure during the wave-free period window. Of note, the iFR algorithm does not perform wave-intensity analysis to detect the patient-specific wave-free period, but uses the definition of 25% of the way into diastole up to 5 ms before the start of systole.
THE iFR AND BASELINE Pd/Pa RELATIONSHIP
The need to assess the wave-free period specifically in order to obtain diagnostically accurate resting measurements has been called into question26. Both the retrospective RESOLVE study and the meticulously conducted prospective ADVISE II study failed to demonstrate superiority of iFR over baseline Pd/Pa calculated over the whole cardiac cycle when compared to FFR27,55,56. While the correlation coefficient between iFR and baseline Pd/Pa is strong (r=0.97; p<0.001 as reported in the RESOLVE study2), it is important to note that iFR and baseline Pd/Pa are not interchangeable. Discordance does occur and divergence between the indices is most pronounced at lower values. The group that proposed iFR stated that it is less sensitive to measurement errors and pressure drift than Pd/Pa, because the resting transstenotic pressure gradient is larger during the wave-free period than during the whole cycle53,57. Furthermore, the values of the baseline Pd/Pa are spread across a narrower range of values when compared to iFR, again suggesting that baseline Pd/Pa is more prone to pressure drift39,58. Further work is needed to determine whether the iFR algorithm is truly needed to perform accurate pressure measurements under resting conditions or whether using the simpler baseline Pd/Pa would suffice. However, the aforementioned discordance between iFR and baseline Pd/Pa means that the upcoming randomised clinical trials investigating iFR do not apply to baseline Pd/Pa. Dedicated studies would need to be undertaken before baseline Pd/Pa could be clinically implemented.
LESION ASSESSMENT IN THE SETTING OF AORTIC VALVULAR STENOSIS
While autoregulation may impact on the iFR results under variable haemodynamic resting conditions, it may conversely ensure that structural cardiac alterations will have a reduced impact on resting in comparison to hyperaemic conditions. Wiegerinck et al recently demonstrated that, in patients undergoing transcatheter aortic valve implantation, the procedure did not alter coronary flow velocity or microvascular resistance under resting conditions in unobstructed coronary arteries59. Under hyperaemic conditions however, coronary flow velocity rose while microvascular resistance declined after the procedure. This was attributed to normalisation of the left ventricular end-diastolic pressure that relieves the extravascular compression of the coronary microvasculature during hyperaemia. Under resting conditions however, autoregulation ensures that the myocardial perfusion remains preserved, irrespective of the left ventricular end-diastolic pressure. This could be important in the context of physiological assessment to indicate concomitant coronary artery bypass grafting in the work-up of surgical aortic valve replacement. FFR could initially be negative secondary to a relatively low flow across the stenosis and become positive after hyperaemic flow increases when the aortic stenosis is relieved. Consequently, concomitant bypass grafting may be “falsely” deferred. Resting modalities such as iFR may be preferable in this setting, because resting flow, and iFR by extension, does not change after relieving aortic stenosis. It must be kept in mind, however, that the expected change in transstenotic pressure gradient in the setting of aortic valve replacement is a theoretical extension of observed changes in coronary flow. Further work is needed to establish the actual FFR and iFR changes definitively in this context.
NON-CULPRIT LESION ASSESSMENT IN THE SETTING OF ACUTE MYOCARDIAL INFARCTION
In the acute phase of ST-segment elevation myocardial infarction, an immediate increase in resting flow, as well as a reduction in hyperaemic flow are observed60. This is not only observed in culprit arteries, but also in non-culprit arteries. Recent clinical trials have shown that rapid revascularisation of obstructed non-culprit arteries in addition to the culprit artery is beneficial to clinical outcomes61-63. Ideally, revascularisation of coronary artery disease is guided by physiological lesion assessment6,7. However, the alterations in coronary flow could theoretically lead to overestimation of non-culprit stenoses by iFR and underestimation by FFR in the acute phase. Baseline flow swiftly restores to definitive values, which may permit accurate assessment by iFR briefly after the event64. Non-culprit hyperaemic flow gradually increases up until six months after acute myocardial infarction64-66. Thereby, revascularisation on the basis of FFR may be deferred in the acute phase, but become indicated if it were measured at a later stage when hyperaemic flow has restored to definitive values. Despite these considerations, a study by Ntalianis et al indicated that FFR in intermediate non-culprit stenoses remained unchanged between the acute moment and approximately one month later67. For iFR, such a study has not yet been conducted. Dedicated studies are needed to establish whether FFR- or iFR-guided revascularisation is preferable in non-culprit revascularisation in the setting of acute myocardial infarction.
FFR AND iFR HYBRID APPROACH
Another proposed approach is to employ a hybrid iFR and FFR strategy. With this strategy, iFR is measured first and, if it is below 0.86, revascularisation is indicated; if it is above 0.93, deferral of revascularisation is indicated. When iFR falls within its diagnostic “grey zone” between 0.86 and 0.93, revascularisation will be guided by FFR measurement and adenosine is administered53. With this hybrid approach, iFR alone is measured in 57 to 68% of cases and adenosine can thus be obviated26,53,55,68,69. Nevertheless, a good classification agreement with FFR only guided revascularisation of 91 to 95% is retained. The hybrid iFR and FFR strategy has been endorsed in the ongoing SYNergy between percutaneous coronary intervention with TAXus and cardiac surgery II (SYNTAX II) study70. This study investigates the treatment of three-vessel disease with contemporary PCI techniques in patients with an intermediate anatomical SYNTAX score. Because procedural times are relatively long in patients with three-vessel disease, the potential to obviate adenosine administration in up to three vessels will significantly expedite the procedure and enhance the feasibility of the study. The SYNTAX II study demonstrates how uptake of physiological interrogation of coronary artery disease may be improved by refraining from the time-consuming process of establishing coronary hyperaemia.

