
Abstract
Aims: Physiological indices such as fractional flow reserve (FFR), instantaneous wave-free ratio (iFR) and resting distal coronary to aortic pressure (Pd/Pa) are increasingly used to guide revascularisation. However, reliable assessment of individual stenoses in serial coronary disease remains an unmet need. This study aimed to compare conventional pressure-based indices, a reference Doppler-based resistance index (hyperaemic stenosis resistance [hSR]) and a recently described mathematical correction model to predict the contribution of individual stenoses in serial disease.
Methods and results: Resting and hyperaemic pressure wire pullbacks were performed in 54 patients with serial disease. For each stenosis, FFR, iFR, and Pd/Pa were measured by the translesional gradient in each index and the predicted FFR (FFRpred) derived mathematically from hyperaemic pullback data. “True” stenosis significance by each index was assessed following PCI of the accompanying stenosis or measurements made in a large disease-free branch. In 27 patients, Doppler average peak flow velocity (APV) was also measured to calculate hSR (hSR=∆P/APV, where ∆P=translesional pressure gradient). FFR underestimated individual stenosis severity, inversely proportional to cumulative FFR (r=0.5, p<0.001). Mean errors for FFR, iFR and Pd/Pa were 33%, 20% and 24%, respectively, and 14% for FFRpred (p<0.001). Stenosis misclassification rates based on FFR 0.80, iFR 0.89 and Pd/Pa 0.91 thresholds were not significantly different (17%, 24% and 20%, respectively) but were higher than FFRpred (11%, p<0.001). Apparent and true hSR correlated strongly (r=0.87, p<0.001, mean error 0.19±0.3), with only 7% of stenoses misclassified.
Conclusions: Individual stenosis severity is significantly underestimated in the presence of serial disease, using both hyperaemic and resting pressure-based indices. hSR is less prone to error but challenges in optimising Doppler signals limit clinical utility. A mathematical correction model, using data from hyperaemic pressure wire pullback, produces similar accuracy to hSR and is superior to conventional pressure-based indices.
Introduction
The functional significance of coronary artery disease (CAD) is frequently assessed during diagnostic angiography using pressure-based indices such as fractional flow reserve (FFR) and instantaneous wave-free ratio (iFR), with growing evidence that physiologically guided management is superior to angiography alone1,2,3,4,5,6. Within elderly and diabetic populations, serial/diffuse CAD is common, being found in up to 40% of vessels at angiography7,8,9. Serial CAD is likely to affect physiological assessment of individual stenoses: any additional resistance will affect the translesional pressure gradient at any point9,10, with hyperaemic indices purported to be particularly vulnerable11.
The techniques most commonly used in serial CAD are FFR or iFR pullback12. Both pressure wire techniques rely on operators assessing the magnitude of trans-stenotic pressure change to decide whether a stenosis is functionally significant. Resting indices in particular make the assumption that the effect of a stenosis is similar in isolation to what it is in the presence of serial disease12. Whilst these pullback techniques are widely used13, there is concern that true stenosis significance is unknown, with misclassification and incorrect revascularisation strategies chosen9.
Recently, a mathematical correction model (FFRpred) has been developed using an in vitro 3D printed model, that improves the accuracy of FFR pullback in predicting true stenosis significance in serial CAD14. This model relied on assuming that serial stenoses behave like resistors in series. Resistance of stenoses can be assessed in the catheterisation laboratory with the pressure and Doppler-based index of hyperaemic stenosis resistance (hSR). Despite the lack of studies to correlate this to clinical outcomes, hSR has been shown to detect reversible perfusion defects seen non-invasively accurately15 and has been proposed as the best stenosis-specific physiological index and an independent reference standard to assess other indices16,17. Despite the inherent strengths of hSR, it has never been assessed in serial CAD, nor has the novel method (FFRpred) been assessed against conventional resting and hyperaemic indices.
This study aimed to assess the accuracy of resting and hyperaemic indices in predicting true stenosis significance in the presence of serial disease. The diagnostic accuracy of hSR was also assessed and compared to conventional pressure-based indices. We subsequently aimed to evaluate a novel pressure wire pullback-based mathematical solution for prospective prediction of individual stenosis significance (FFRpred) in serial CAD.
Methods
STUDY POPULATION
Patients undergoing elective angiography and/or PCI for stable angina were enrolled and chosen based on having two stenoses (>30% diameter stenosis by quantitative coronary angiography [QCA]) separated by ≥10 mm of normal segment, where the operator would consider treating them separately. Exclusion criteria were previous coronary artery bypass grafting (CABG), recent acute coronary syndrome (<4 weeks) and significant valvular disease. All patients were enrolled at Guys and St Thomas’ NHS Trust and provided written informed consent. Ethical approval was obtained from the UK Health Research Authority and the local Research Ethics Committee (Ref: 15/LO/2011).
CATHETERISATION LABORATORY STUDY PROTOCOL
Coronary angiography was performed via the radial or femoral artery using a standard Judkins technique. All patients received prior loading with two antiplatelet drugs and intra-arterial heparin to maintain an activated clotting time greater than 250 s. Intracoronary isosorbide dinitrate (500 mcg) was administered for all pressure wire recordings.
PRESSURE WIRE PULLBACK MEASUREMENTS
Guidewire pressure sensors were normalised in the aorta before being advanced, with the distal wire position documented fluoroscopically so that subsequent measurements were made from the same position. A fixed rate manual pressure wire pullback (at ~1 mm/s without stopping to check fluoroscopic position) was performed from the distal-proximal during IV adenosine-induced hyperaemia (140 mcg/kg/min), with onset of hyperaemia confirmed using invasive pressure waveform changes18. We allowed for stabilisation of FFR by ≥30 seconds after the “smart minimum FFR” had been reached (lowest 5-beat average19). After ≥60 seconds after hyperaemia (to allow for a return to baseline conditions), the vessel was re-wired and pullback repeated in resting conditions. The change in iFR and FFR across each stenosis was termed ∆iFRapp and ∆FFRapp, respectively. ∆Pd/Pa was also calculated post hoc from the resting pullback. Hyperaemic and resting pressure wire measurements were made using a Philips Volcano wire in order to measure iFR using proprietary methods.
PRESSURE/DOPPLER MEASUREMENTS
In a subset of patients in whom operators judged that pre- and post-isolation measurements with a 0.014” dual pressure-Doppler sensor guidewire were feasible (ComboWire® 9500; Philips Volcano, San Diego, CA, USA, with 0 cm pressure and Doppler sensor offset), simultaneous Doppler-averaged peak flow velocity (APV, cm/s) measurements were also made. Measurements were taken during intracoronary adenosine-induced hyperaemia (100 mcg for left; 60 mcg for right), beyond the distal stenosis, midway between stenoses and before the proximal stenosis, with Doppler signals optimised at each stage. Intracoronary adenosine was used to enable optimisation of Doppler signals without having to continue IV adenosine for several minutes, with evidence suggesting no difference in FFR measured by either mode of adenosine delivery20. This enabled us to generate translesional values for resistance (hyperaemic stenosis resistance, mmHg/cm·s, hSR = (Pa-Pd)/APV during hyperaemia21). Cases where clear Doppler traces were not obtained were excluded from analysis.
IDENTIFYING TRUE VALUES OF STENOSIS PHYSIOLOGICAL SIGNIFICANCE
To compare apparent and true physiological measurements, one of two methods was used: 1) pressure wire pullback and pressure-Doppler measurements repeated following PCI of one of the two stenoses (the treated lesion was chosen by the operator using standard practice); 2) pressure wire pullback and pressure-Doppler measurements repeated in a large disease-free daughter vessel (>2.5 mm in diameter) after assessing the serially diseased vessel, for example, left main coronary artery (LMCA) stenosis with accompanying left anterior descending (LAD) serial disease, but with an unobstructed left circumflex (LCx) artery. This method has previously been shown to be reliable when assessing LMCA ∆FFRtrue provided the total FFR of the serially diseased vessel is >0.4522. Figure 1 summarises the protocol.
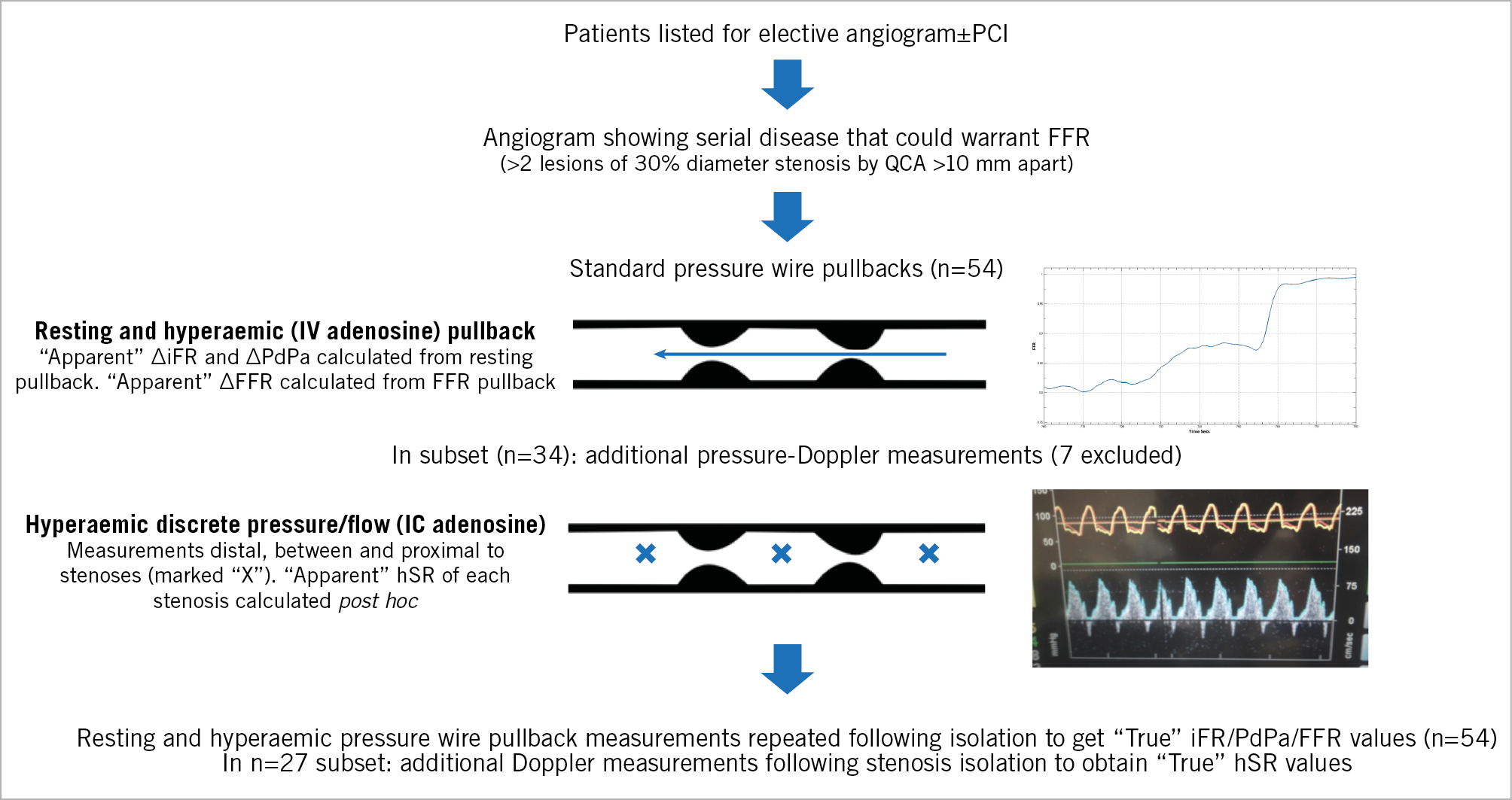
Figure 1. Summary of catheterisation laboratory protocol.
DEVELOPING AN ALGORITHM TO PREDICT FFRtrue (FFRpred)
Details are provided in Supplementary Appendix 1 and in the original manuscript by Modi et al, 201814. To utilise this equation, a conventional manual FFR pullback manoeuvre is sufficient. Typically, a further 30 seconds is needed for inputting the pullback data into the correction equation (a step being automated onto a pressure wire platform). It is important to note that “FFRpred” in our study is different from “FFRpred” as described by De Bruyne et al23, whose equation required measurement of coronary occlusive pressure.
STATISTICAL ANALYSIS
The sample size was chosen based on power calculations to ensure ≥80% power according to a continuous outcome equivalence study. Data analysis was performed using SPSS, Version 23 (IBM Corp., Armonk, NY, USA). Normality was visually assessed (using histograms and Q-Q plots). Continuous data were expressed as mean±standard deviation and compared using paired t-tests. A two-tailed test of significance was performed for all analyses, with p<0.05 considered statistically significant. Categorical variables were assessed using a chi-squared test. Correlations were assessed using Pearson’s R correlation coefficients.
Results
Fifty-four patients with serial CAD had apparent and true values calculated for various pressure-based indices. In 34 patients, combined pressure-Doppler measurements were made to determine hSR before and after lesion isolation. Seven cases were excluded as clear Doppler traces could not be obtained (difficulty in obtaining clear Doppler traces is a major limitation of measuring hSR outside academic settings). The cumulative vessel FFR of the 54 vessels was 0.72±0.10, iFR 0.8±0.13 and resting Pd/Pa 0.87±0.08. In 20/54 cases the proximal lesion was isolated, with the majority of cases isolated by pre-/post-PCI measurements (Table 1 for breakdown). There was no difference in results based on which stenosis was in question. In 7% of cases (4/54) where pressure-only measurements were made, recordings were made using non-proprietary guidewires and consoles due to operator preference. To preserve consistency of iFR measurements, post hoc iFR extraction was not performed in these cases. Baseline demographics and procedural details are summarised in Table 1.
PRESSURE-BASED INDICES IN SERIAL DISEASE
For FFR, significant underestimation of lesion severity occurred, regardless of whether the proximal or distal lesion was considered, with a mean difference between FFRapp and FFRtrue of 0.05±0.05 (p<0.001). This corresponded to a mean error, as a proportion of the true translesional FFR of 33% (Figure 2). The extent of lesion underestimation was found to be inversely proportional to total vessel FFR: the lower the total FFR, the greater the error (R=0.50, p<0.001).
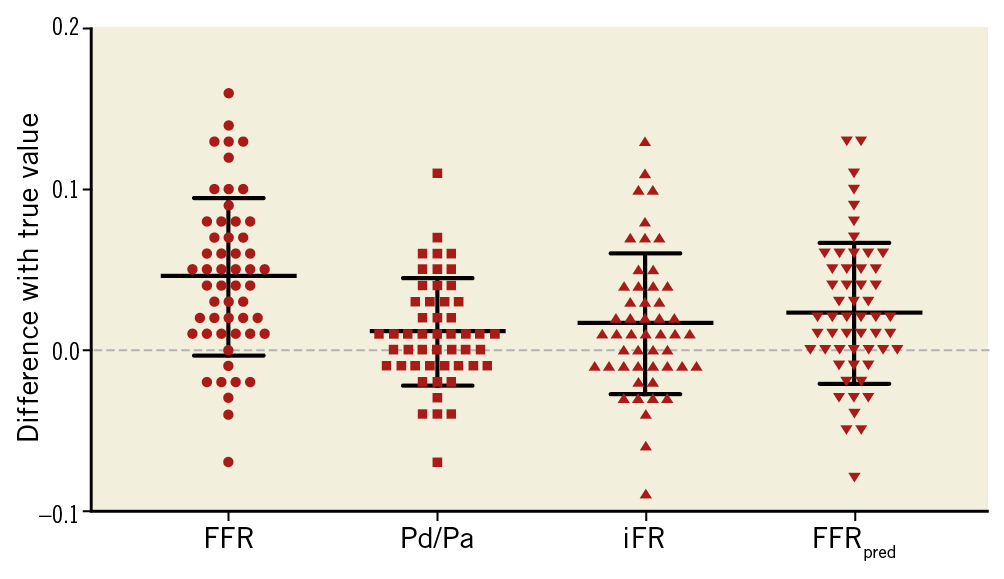
Figure 2. Scatter plots demonstrating the error in estimating true stenosis severity using different indices (data points above zero line=lesion underestimation). For FFR, iFR and resting Pd/Pa, there was significant (p<0.01) underestimation with a mean difference between apparent and true values of 0.05±0.05, 0.02±0.04 and 0.01±0.03, respectively. This corresponded to a mean error, as a proportion of the true change in FFR, iFR and Pd/Pa across each lesion, of 33%, 20% and 23%, respectively. With FFRpred, the error was significantly reduced (0.02±0.04, corresponding to a 14% error).
For iFR and resting Pd/Pa, there was also significant underestimation with a mean difference between apparent and true values of 0.02±0.04 and 0.01±0.03, respectively. This corresponded to a mean error, as a proportion of the true translesional gradient, of 20% and 23%, respectively. For the mathematical solution, FFRpred, the absolute underestimation was 0.02±0.04, which corresponded to a mean relative error of 14% (Figure 2).
RESISTANCE INDICES IN SERIAL DISEASE
Translesional hSR values when a stenosis was present alongside another stenosis (hSRapp) was found to correlate strongly with the hSR of the stenosis in isolation (hSRtrue), p<0.001, R=0.87. Mean absolute error for hSR was 0.19±0.3 mmHg/cm·s, which corresponded to a mean relative error of 26%, as a proportion of true hSR. In addition, we found that the sum of translesional hSR values for each lesion was different from the total hSR in the vessel (Figure 3), although this did not meet statistical significance (p=0.07).
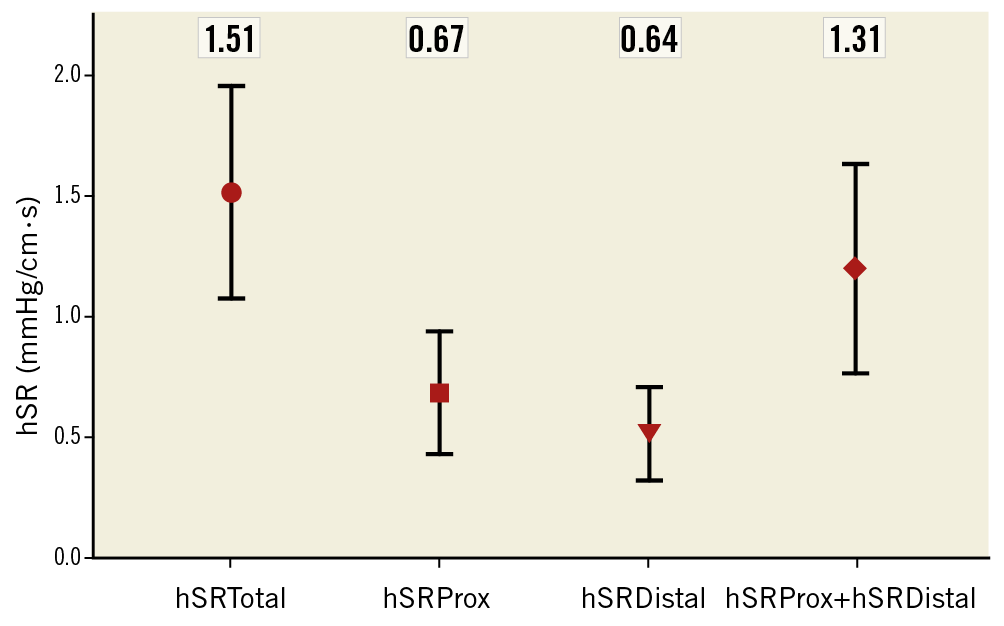
Figure 3. Chart plotting hSR of proximal stenosis, distal stenosis, sum of both and total vessel hSR. Values given as mean±standard deviation; hSR units mmHg/cm·s. The mean sum of the hSR for both stenoses was not significantly different from total hSR (p=0.07).
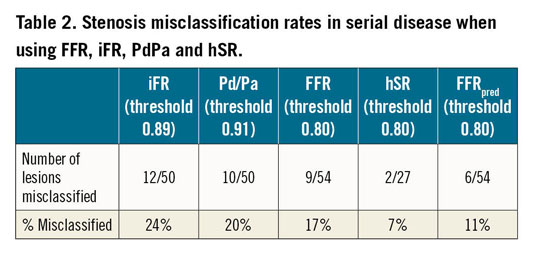
Using cut-off values of FFR 0.8, iFR 0.89, PdPa 0.91 and hSR 0.8 to identify functionally significant stenoses, we established misclassification rates for lesion-attributable FFR, iFR, PdPa and hSR (Table 2), and showed that they were not significantly different. With FFRpred, there was a significant reduction in misclassification (p<0.001) with only 11.1% of stenoses misclassified. There was no observed difference in the accuracy of FFRpred between LAD and non-LAD lesions, although we acknowledge the small number of RCA/LCx measurements (Table 1).
Discussion
The main findings are as follows:
i) The translesional gradient of both resting and hyperaemic pressure-based indices leads to significant lesion underestimation in serially diseased vessels. Using conventional thresholds, 17-24% of lesions are misclassified, depending on the index used. The lower the total vessel FFR/iFR, the greater the underestimation.
ii) A Doppler-based hyperaemic resistance index (hSR) results in less misclassification but Doppler traces of sufficient quality could not be obtained in ~20% of cases (7/34).
iii) A novel mathematical solution to predict true lesion-attributable FFR (FFRpred) significantly reduces misclassification.
Importantly, our study is the first to compare the accuracy of different physiological indices in serial CAD. It is also the only study to test the value of pressure flow-based resistance indices in serial CAD and the first to validate a novel mathematical solution clinically, using only inputs derived from routine hyperaemic pressure wire pullback.
EXISTING DATA OF PRESSURE-DERIVED INDICES IN SERIAL CAD
De Bruyne et al have previously demonstrated that FFR cannot be reliably applied to predict the FFR of individual stenoses present in series, with significant errors demonstrated in their seminal studies10,23. Whilst their data suggest that the proximal stenosis is more prone to error in the presence of a distal stenosis than vice versa, our data show that there is no significant difference. De Bruyne et al subsequently derived a complex formula requiring measurement of coronary occlusive wedge pressure. Whilst well validated, the method is rarely used and considered impractical24. Furthermore, the absolute FFR error reduction quoted in their study (0.029±0.004) is similar to the improvement in error from the correction equation validated in our study24.
In the absence of a definitive FFR solution, iFR pullback is increasingly used, with proponents arguing that stenosis interaction is minimal at rest. To validate this method, Nijjer et al assessed 32 serially diseased vessels to show a mean difference between iFRapparent (“ΔiFRexp” in their study) and iFRtrue (“ΔiFRobs”) of 0.016±0.00412. Nijjer et al argue that this error is small (we demonstrated a similar absolute error in our study, 0.02±0.04). Given the smaller range of resting ΔP values for iFR, a 0.016 change has potentially greater importance (the 0.02±0.04 iFR error in our study represents a nearly 20% relative error with 24% of stenoses misclassified). A more recent study by Kikuta et al (iFR-GRADIENT)25 found a smaller error (0.011±0.004), although this still represented a relative error of 14%. Whilst this supports the hypothesis that there is greater error with uncorrected hyperaemic indices, it is still significant with no comments regarding stenosis misclassification.
Overall, our data show that all pressure-derived indices are prone to significant error in serial CAD. Pd/Pa and iFR both appear to perform better in terms of relative error; however, given the lower spatial resolution of these resting indices (i.e., 0.1 error in iFR is more significant than the same for FFR), they appear to result in similarly marked stenosis misclassification.
RESISTANCE INDICES IN SERIAL CAD
Our data demonstrate that the translesional resistance of a stenosis can be considered largely independent of disease elsewhere, with apparent and true translesional hSR correlating strongly. Whilst correlating strongly, the sum of hSR values is lower than total vessel hSR (albeit not reaching statistical significance) (Figure 3). This may be due to the contribution of normal coronary segments between lesions to total resistance, in addition to the effect of side branches and collateral flow. In addition, it has been reported that hSR may be misleading when microcirculation is disrupted26. Nonetheless, hSR does appear to be more robust than pressure-derived indices in predicting stenosis significance with only 7.4% of stenoses misclassified (Table 2). Universal utilisation of such indices is however limited by challenges of obtaining clear intracoronary Doppler traces.
VALIDATION OF A NOVEL MATHEMATICAL SOLUTION IN SERIAL CAD
A major assumption underpinning the novel solution (FFRpred) was that it is reasonable to use an Ohm’s law equivalent to describe the relationship between trans-stenotic pressure and flow. This assumption was supported by measurements of total vessel and translesional hSR values (Figure 1), showing the relative independence of translesional hSR values that justifies not needing to measure coronary occlusive pressure. The equation was derived in phantoms without major intervening side branches and, whilst not reaching significance, the finding that total vessel hSR trends towards being greater than the sum of translesional hSR values does suggest that side branch and collateral flow between stenoses may be influential to an extent. Whilst imperfect, the use of the correction model provides a pragmatic method to correct for errors from pressure wire pullback that reduces the number of misclassified stenoses within a clinical cohort.
CLINICAL IMPLICATIONS AND FUTURE DIRECTION
Our study demonstrates that both hyperaemic and resting physiological assessment of individual stenoses is prone to significant error in serial CAD, particularly when total FFR/iFR is low. Pressure flow-based resistance indices are less prone, but utilisation is limited by difficulties in obtaining intracoronary Doppler traces.
Our study also validates an easily applicable correction equation (FFRpred), relying only on hyperaemic pressure pullback measurements. We acknowledge that, despite this correction, there is a 14% relative error and an 11% stenosis misclassification rate. Whilst imperfect, this improves errors from current iFR/FFR pullback methods. The true clinical utility of this solution now needs to be assessed in a prospective multicentre utility study.
Limitations
Identifying a reference standard against which to compare physiological indices in serial CAD is challenging. We used the translesional change in index when present in isolation, with lesions isolated by PCI or a disease-free side branch. Whilst these methods are established, errors are still possible: the post-PCI effects on microvascular tone have not yet been established27 and the disease-free side branch method (validated when serial disease involves the LMCA) is prone to small errors, particularly with severe disease in the main serially diseased vessel22. This is a relatively small study, albeit the largest to compare existing and novel strategies in the physiological assessment of serial CAD. A larger study in a broader range of serial CAD, together with an assessment of clinical utility, would be an important next step.
Conclusions
Pressure-derived indices, both resting and hyperaemic, significantly underestimate stenosis significance in serial disease, with discrepancy proportional to cumulative disease burden. Pressure flow-based resistance indices are less prone to this error, but universal utilisation is limited by difficulties in obtaining intracoronary Doppler traces. Based on these findings, we have validated a novel mathematical solution to use with routine hyperaemic pressure wire measurements that significantly reduces the error of pressure-derived indices to a similar extent and should be considered in routine clinical cases of serial CAD.
|
Impact on daily practice Individual stenoses can be underestimated when using hyperaemic and resting pressure wire pullback methods in serial CAD, particularly when cumulative FFR/iFR is low. Doppler-based resistance indices are less prone to error, but universal utilisation will be limited. A novel solution to use with routine hyperaemic pressure wire measurements has been validated and should be incorporated into future pressure wire platforms and assessed in larger studies. |
Acknowledgements
Firstly, we thank the patients who took part. We also thank our catheterisation laboratory and scheduling staff. In addition, we thank the BHF for their ongoing support of UK cardiovascular research.
Funding
Authors B. Modi, H. Rahman, and M. Ryan are funded by British Heart Foundation (BHF) Clinical Research Training Fellowships (FS/15/78/31678, FS/16/49/32320 and FS/18/16/33396, respectively). D. Perera receives support from the NIHR Biomedical Research Centre award to King’s College London.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

