Abstract
Aims: We sought to compare the diagnostic accuracy of basal stenosis resistance index (BSR), instantaneous wave-free ratio (iFR) and fractional flow reserve (FFR) for stenosis-specific myocardial ischaemia identified by means of a combined reference standard of myocardial perfusion scintigraphy and the hyperaemic stenosis resistance index.
Methods and results: BSR and FFR were determined for 299 coronary stenoses, iFR was determined for 85 coronary stenoses (iFR cohort). The discriminative value for stenosis-specific myocardial ischaemia was compared by means of the area under the receiver operating characteristic (ROC) curves (AUC). Classification agreement with the reference standard was determined according to ROC curve-derived ischaemic cut-off values, as well as according to clinical cut-off values, equivalent to the 0.80 FFR cut-off. Across all stenoses, the discriminative value of BSR and FFR was equivalent (AUC: 0.90 and 0.91, respectively, p=0.46). In the iFR cohort, the discriminative value was equivalent for BSR, iFR, and FFR (AUC: 0.88, 0.84, and 0.88, respectively; p≥0.20 for all). At both ischaemic as well as clinical cut-off values, classification agreement with the reference standard was equivalent for BSR and FFR across all stenoses, as well as for BSR, iFR, and FFR in the iFR cohort.
Conclusions: BSR, iFR, and FFR have equivalent diagnostic accuracy for the detection of ischaemia-generating coronary stenoses.
Introduction
In the management of patients with stable coronary artery disease, fractional flow reserve (FFR)-guided revascularisation is associated with significant clinical benefit, as well as an important reduction in healthcare costs compared to angiographic guidance1-6. Nonetheless, its adoption in clinical practice remains low7,8, which is considered to result from a combination of financial disincentive and practical ambiguities associated with the use of FFR in daily clinical practice. Among the latter, the requisite to administer potent vasodilators9-11, such as adenosine, is considered an important denominator12. The basal stenosis resistance index (BSR)13, and the instantaneous wave-free ratio (iFR)14-16 are novel physiological indices that are determined during basal conditions, and which have demonstrated a potential to identify haemodynamically severe stenoses without concomitant administration of adenosine. Differently from BSR, which was initially validated against myocardial perfusion scintigraphy (MPS)13, iFR was validated using FFR as a comparator14,16. The latter fact impedes a comparison of the diagnostic efficiency of BSR and iFR, since FFR itself is a surrogate for non-invasive detection of inducible myocardial ischaemia17.
The objective of the present study was to perform a head-to-head comparison of BSR, iFR, and FFR in the detection of stenosis-induced myocardial ischaemia, using a combined reference standard comprising MPS and an intracoronary-derived index of stenosis resistance, the hyperaemic stenosis resistance index (HSR)18-23, that allowed identification of cases in which myocardial ischaemia was present and most likely a direct result of the epicardial stenosis.
Methods
DATA SOURCE
Between April 1997 and September 2006, a total of 228 stable coronary artery disease patients, referred for intracoronary evaluation of at least one intermediate coronary artery stenosis (40%-70% diameter stenosis on visual angiographic assessment), were included. We excluded patients with ostial stenoses, ≥2 stenoses in the same coronary artery, severe renal function impairment (MDRD calculated glomerular filtration rate <30 mL/min/1.73 m2), significant left main coronary artery stenosis, atrial fibrillation, recent myocardial infarction (<6 weeks before screening), prior coronary artery bypass graft surgery, or visible collateral development to the perfusion territory of interest. The institutional ethics committee approved the study protocol and all patients gave written informed consent.
MYOCARDIAL PERFUSION SCINTIGRAPHY
Myocardial perfusion scintigraphy (MPS) was performed in all patients with the use of either Technetium 99m-labelled sestamibi (MIBI) or tetrofosmin (Myoview; GE Healthcare, Little Chalfont, Buckinghamshire, UK) according to a two-day stress-rest protocol. Stress was induced either pharmacologically by adenosine or dipyridamole, or by exercise. Defect reversibility and localisation were determined by a panel of experienced nuclear medicine physicians, blinded to the angiographic and intracoronary haemodynamic data. Perfusion defects were classified semi-quantitatively as dubious, mild, moderate or severe, and improvement at rest of more than one grade was considered to be a “reversible” perfusion defect. Improvement of just one grade or no improvement was considered to be a “persistent” perfusion defect. The result was considered positive when a reversible perfusion defect was allocated to the perfusion territory of the coronary artery of interest.
CARDIAC CATHETERISATION AND INTRACORONARY HAEMODYNAMIC MEASUREMENTS
All patients underwent cardiac catheterisation within one week after MPS. At the time of angiography and intracoronary measurements, the operator was blinded to the results of the MPS study. Angiographic images were obtained in a manner suitable for quantitative analysis. Quantitative coronary angiography analysis was performed offline to determine percent diameter stenosis by means of a validated automated contour detection algorithm (QCA-CMS Version 3.32; Medis, Leiden, The Netherlands). Intracoronary pressure was measured distal to the target lesion with a 0.014 inch pressure-monitoring guidewire (Volcano Corp., San Diego, CA, USA). Coronary blood flow velocity measurements were performed directly after pressure measurements using a 0.014 inch Doppler-tipped guidewire (Volcano Corp.). Pressure and flow velocity measurements were performed both during basal conditions as well as during hyperaemia induced by an intracoronary bolus of adenosine (20 μg-40 μg).
DATA ANALYSIS
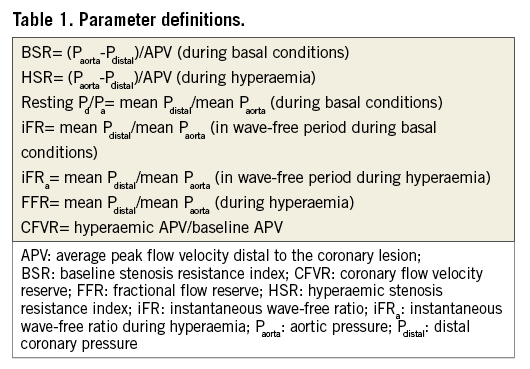
Per beat averages of coronary pressure and flow velocity were available in 299 coronary stenoses. Raw pulsatile coronary pressure tracings for calculation of iFR, obtained at least 30 seconds after last contrast medium injection, were available in 85 out of 299 coronary stenoses. Haemodynamic indices derived from per beat averages included resting distal coronary to aortic pressure ratio (resting Pd/Pa), FFR, coronary flow velocity reserve (CFVR), HSR, and BSR. In the available raw pulsatile data, iFR as well as iFR during adenosine administration (iFRa) were calculated in a blinded fashion using the fully automated iFR algorithm developed at Imperial College London to detect the iFR window, as reported previously16. The definitions of the evaluated parameters are summarised in Table 1.
COMBINED REFERENCE STANDARD CLASSIFICATION
The MPS study was considered positive when a reversible perfusion defect was allocated to the perfusion territory of the coronary artery of interest. HSR was considered positive when the stenosis resistance during hyperaemia was greater than 0.80 mmHg cm−1 sec18,23. Stenoses were considered to be ischaemia-generating only when both MPS and HSR were positive. As such, the reference standard comprehensively included both the presence of perfusion maldistribution as assessed by MPS, and its origin in the epicardial stenosis as identified by HSR.
Statistical analysis
Receiver operating characteristic (ROC) curves were used to compare the discriminative power of BSR, iFR, iFRa, resting Pd/Pa, and FFR for the presence of stenosis-related perfusion defects by comparison of the area under the curve (AUC). Subsequently, for each parameter, classification agreement with reference standard outcomes was determined according to two separate cut-off values. First, classification agreement was determined according to the ischaemic cut-off value for stenosis-specific myocardial ischaemia on the combined reference standard. The optimal ischaemic cut-off values were defined as the cut-off values with the highest sum of sensitivity and specificity for a positive classification on the combined reference standard. Second, classification agreement for each parameter was determined according to its clinically applied cut-off value, indicating a cut-off value comparable with the clinically adopted FFR 0.80 cut point: 0.80 for FFR2,5, and 0.90 for iFR24. In the absence of a clinically adopted cut-off value for BSR, a data-derived optimal clinical cut-off value was identified as the cut-off value with the highest sum of sensitivity and specificity for FFR ≤0.80. Overall classification agreement with the reference standard of all parameters was compared using the McNemar test. All analyses were performed both within the complete data set (full cohort) to compare the diagnostic performance of BSR, resting Pd/Pa, and FFR, as well as within the data set in which iFR values were available (iFR cohort) to compare the diagnostic performance of BSR, iFR, iFRa, resting Pd/Pa, and FFR. As a sensitivity analysis, ROC curves were constructed and AUC comparison was performed using MPS only as the reference standard, both in the full cohort as well as within the iFR cohort. In this analysis, ROC curves were constructed for HSR as well, for which the comparison against BSR and FFR has been previously reported13.
The dependence of BSR and iFR on heart rate and blood pressure during basal conditions was evaluated by evaluating the association of the relative difference of BSR compared with HSR (BSR-HSR/HSR), and of iFR compared with FFR (iFR-FFR/FFR), with heart rate and rate pressure product during basal conditions, by means of linear regression analysis25. Unimodality of the FFR value distribution was assessed using the Hartigan’s dip test. Continuous variables were expressed as mean (±SD) or median (25th-75th percentile) and comparison was performed using the Student’s t-test or Mann-Whitney U test where appropriate. Categorical variables were presented as frequencies (percentage) and compared with the χ2 test. A p-value <0.05 (two-sided) was considered statistically significant.
Results
PATIENTS
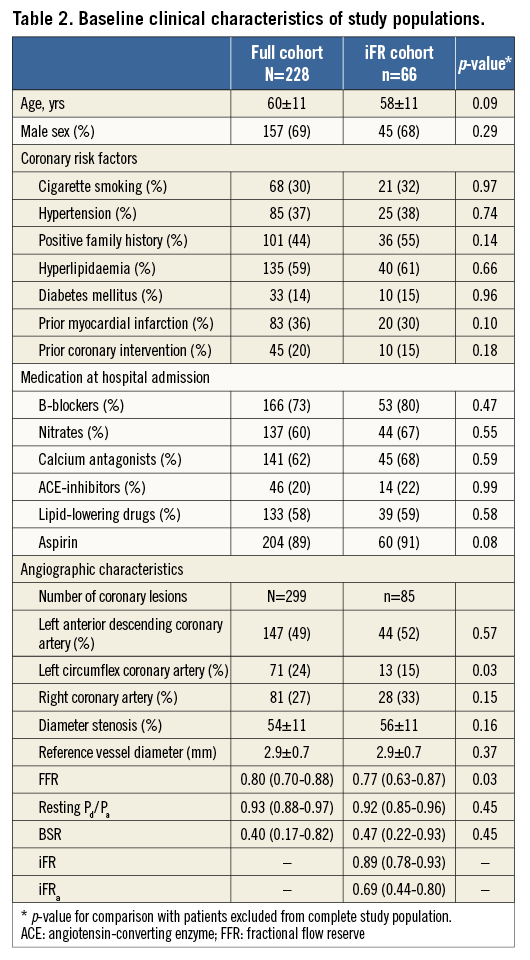
Baseline clinical characteristics of the study population are shown in Table 2. Although the stenoses belonging to the iFR cohort presented slightly lower FFR values, and were less frequently located in the left circumflex coronary artery, there were no pertinent differences with the full cohort.
PHYSIOLOGICAL STENOSIS CHARACTERISTICS
Stenosis severity distribution by FFR showed a unimodal distribution, both in the full cohort, as well as within the iFR cohort, with a preponderance of stenoses of intermediate physiological severity: approximately 65% of FFR values fell between 0.6 and 0.9 (Figure 1A, Figure 1B). The Hartigan’s dip test confirmed the unimodality of the data (dip test=0.02, p=0.52 for the full cohort, and dip test=0.03, p=0.9 for the iFR cohort).
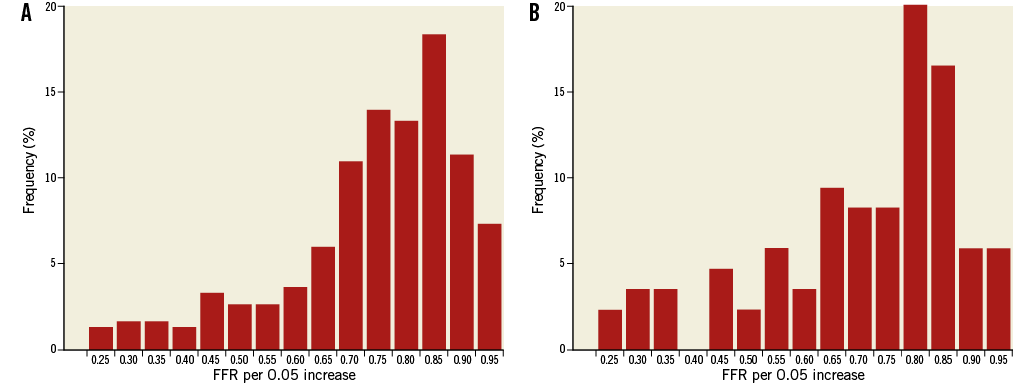
Figure 1. Lesion severity distribution by fractional flow reserve in: A) the full cohort (N=299), and B) the iFR cohort (n=85). Lesion distribution shows a unimodal distribution in both study populations, predominantly true intermediate coronary lesions with an FFR between 0.6 and 0.9.
The relative error of BSR compared with HSR was not associated with either heart rate (r2=0.010, p=0.18) or rate pressure product (r2=0.006, p=0.21) during basal conditions. Similarly, the relative error of iFR compared with FFR was not associated with either heart rate (r2=0.018, p=0.23) or rate pressure product (r2<0.001, p=0.91) during basal conditions.
Diagnostic performance of BSR and FFR within the full cohort (N=299)
RECEIVER OPERATING CHARACTERISTIC CURVE ANALYSIS
In the full cohort, ROC curve analysis yielded an equivalent AUC for stenosis-specific myocardial ischaemia for BSR and FFR (BSR AUC: 0.90 vs. FFR AUC: 0.91, p=0.47; Figure 2A, Table 3). The AUC of resting Pd/Pa for stenosis-specific ischaemia was lower compared with both BSR and FFR (resting Pd/Pa AUC: 0.87; p≤0.02 for both comparisons; Figure 2A, Table 3).
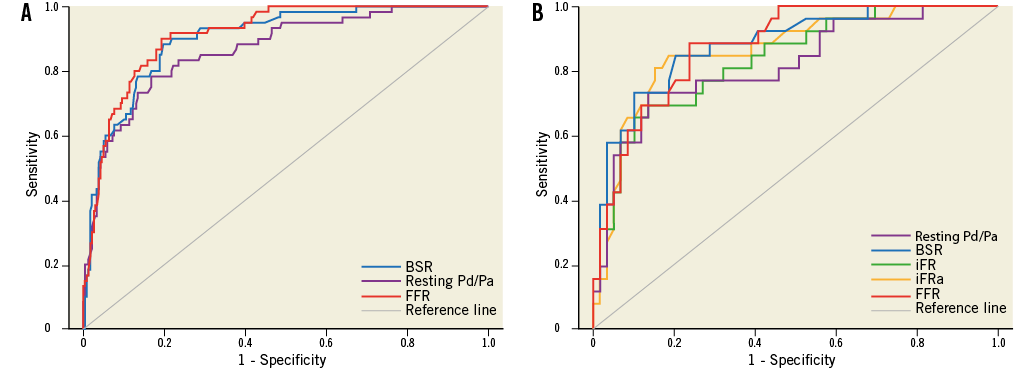
Figure 2. Receiver operating characteristic curves using a combined reference standard of myocardial perfusion scintigraphy and hyperaemic stenosis resistance index for: A) BSR, resting Pd/Pa, and FFR within the full cohort (N=299), and B) BSR, iFR, iFRa, resting Pd/Pa, and FFR within the iFR cohort (n=85).
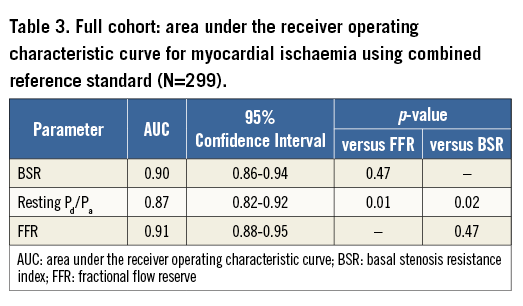
The optimal ischaemic cut-off values within the full cohort were 0.66 mmHg cm−1 sec for BSR (sensitivity 88.3%, specificity 80.3%), and 0.75 for FFR (sensitivity 91.7%, specificity 78.7%). The optimal clinical cut-off value for BSR (to match an FFR of 0.80) was 0.47 mmHg cm−1 sec.
CLASSIFICATION AGREEMENT
Out of 299 coronary stenoses, the reference standard classified 60 (20%) as true positive, and 184 (62%) as true negative, while 55 (18%) were considered indeterminate because of disagreement in terms of MPS and HSR classification.
Overall classification agreement, as well as positive predictive value and negative predictive value, was equal between BSR and FFR at their respective ischaemic cut-off values (Table 4). Moreover, classification agreement with the reference standard was also equivalent between BSR and FFR at their respective clinical cut-off values (Table 4).
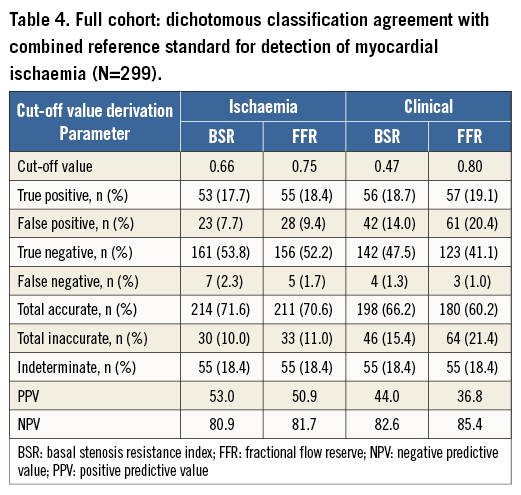
For both BSR and FFR, changing the dichotomisation cut-off from ischaemic to clinical was associated with an increase in the number of false-positive test outcomes (an increase of 6.3% for BSR, and 11% for FFR), while it did not lead to a relevant decrease in false-negative test outcomes (a decrease of 1% for BSR, and 0.7% for FFR), resulting in an increase of inaccurately classified stenoses (an increase of 5.4% for BSR, and 10.4% for FFR; Table 4).
Diagnostic performance of BSR, iFR, and FFR within the iFR cohort (n=85)
RECEIVER OPERATING CHARACTERISTIC CURVE ANALYSIS
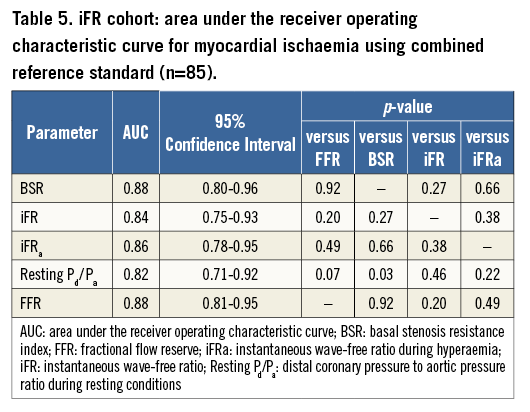
Within the iFR cohort (n=85), BSR, iFR, and FFR had equivalent AUC by ROC curve analysis (AUC 0.88, 0.84 and 0.88, respectively, p≥0.20 for all; Figure 2B, Table 5). Notably, the administration of adenosine did not increase the AUC of iFR (iFRa AUC: 0.86; p>0.05 for all comparisons; Figure 2B, Table 5). The AUC of resting Pd/Pa was only significantly lower than that of BSR (resting Pd/Pa AUC: 0.82 vs. BSR AUC: 0.88, p=0.03, and p>0.05 compared with both iFR and FFR; Figure 2B, Table 5).
The optimal ischaemic cut-off values within the iFR cohort were 0.66 mmHg cm−1 sec for BSR (sensitivity 84.6%, specificity 79.7%), 0.82 for iFR (sensitivity 69.2%, specificity 88.1%), and 0.75 for FFR (sensitivity 88.5%, specificity 76.3%). The clinical cut-off values were predefined and amounted to 0.47 mmHg cm−1 sec for BSR, 0.90 for iFR, and 0.80 for FFR.
CLASSIFICATION AGREEMENT
Within the iFR cohort, the combined reference standard identified 26 out of 85 coronary stenoses (31%) as true positive, 46 (54%) as true negative, and 13 (15%) were considered indeterminate due to MPS and HSR disagreement.
Dichotomous classification agreement with the reference standard was equal between BSR, iFR and FFR at their respective ischaemic cut-off values (Table 6). Moreover, at their respective clinical cut-off values, classification agreement with the combined reference standard was also equal among the three parameters (Table 6).
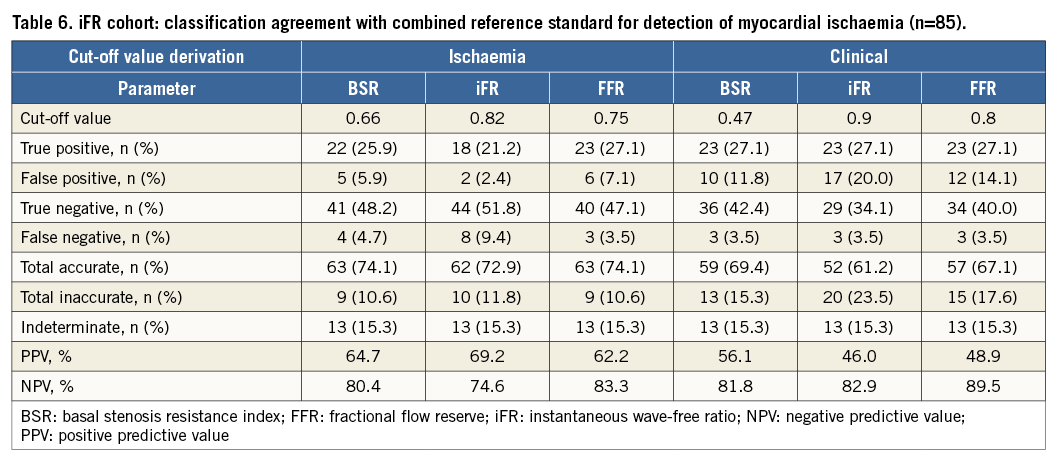
Again, classification by means of the clinical cut-off values yielded an increase in false-positive test outcomes compared with the use of the ischaemic cut-off values (an increase of 5.9% for BSR, 17.6% for iFR, and 7% for FFR), while leading to a limited decrease in false-negative outcomes (a decrease of 1.2% for BSR, 5.9% for iFR, and 0% for FFR), resulting in an increase of inaccurately classified stenoses (an increase of 4.7% for BSR, 11.7% for iFR, and 7% for FFR) (Table 6).
DIAGNOSTIC PERFORMANCE AGAINST MYOCARDIAL PERFUSION SCINTIGRAPHY REFERENCE STANDARD
Using MPS as the reference standard, similar findings and conclusions applied, although, within the full cohort, the numerical difference between BSR or FFR and resting Pd/Pa did not reach statistical significance, and the AUC for HSR was significantly greater than that of BSR, FFR and resting Pd/Pa (Figure 3, Table 7). Within the iFR cohort, the numerical difference between either BSR, FFR, or HSR and resting Pd/Pa only reached statistical significance for the comparison with HSR, and the numerical difference in AUC between HSR and iFRa or FFR marginally missed statistical significance (Figure 4, Table 8).
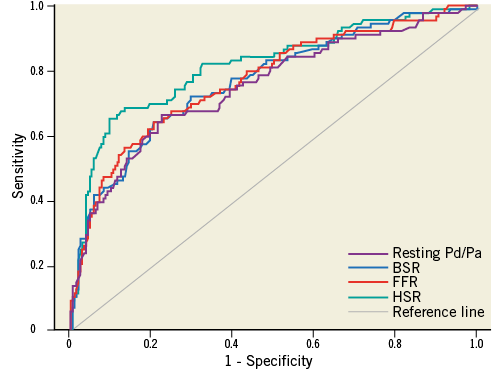
Figure 3. Receiver operating characteristic curves against myocardial perfusion scintigraphy as the reference standard for BSR, resting Pd/Pa, and FFR within the full study cohort (N=299).
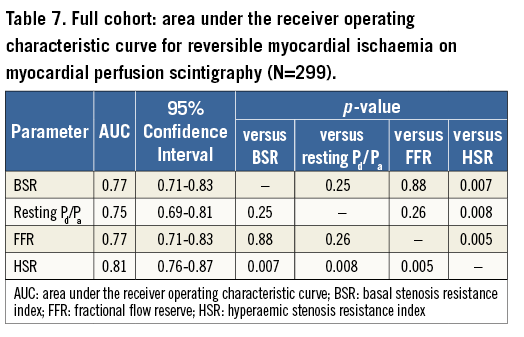
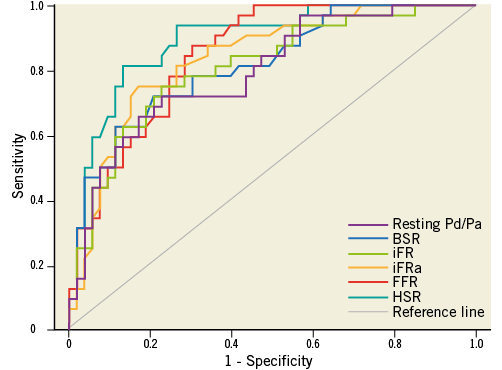
Figure 4. Receiver operating characteristic curves against myocardial perfusion scintigraphy as the reference standard for BSR, iFR, iFRa, resting Pd/Pa, and FFR within the iFR cohort (N=85).
DISCORDANCE BETWEEN FFR AND BSR OR iFR AT THEIR ISCHAEMIC CUT-OFF VALUES IN THE iFR COHORT
BSR disagreed with FFR in 15 (18%) cases, in which BSR was concordant with both MPS and HSR in seven of 15 cases (47%), and FFR was concordant with both MPS and HSR in seven of 15 cases (47%); MPS and HSR were in disagreement in one of 15 cases (7%) (Figure 5A). Out of the 15 discordant cases, BSR and FFR agreed with CFVR in seven (47%) and 8 (53%) cases, respectively (Figure 5A).
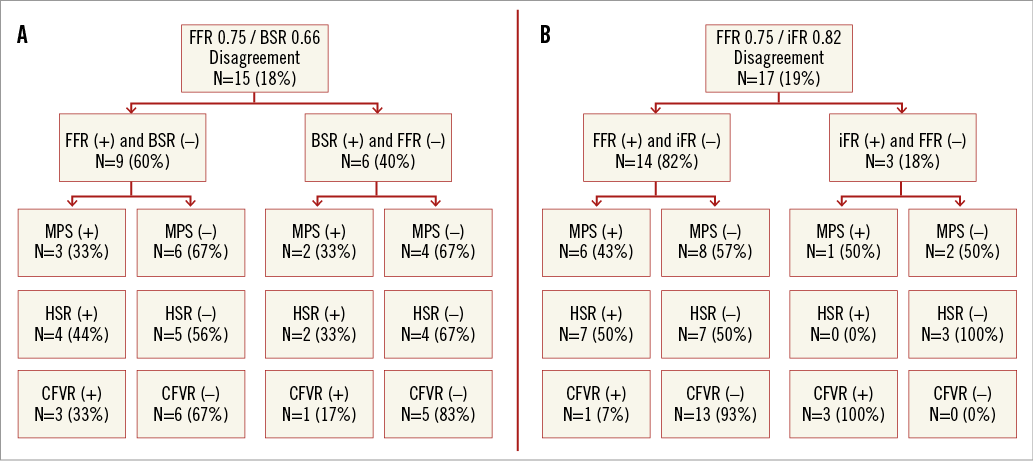
Figure 5. Discordance between iFR/BSR and FFR within the iFR cohort (n=85). A) Between BSR and FFR, and B) between iFR and FFR.
iFR disagreed with FFR in 17 (19%) cases, in which iFR was concordant with both MPS and HSR in six of 17 cases (35%), and FFR was concordant with both MPS and HSR in seven of 17 cases (41%); MPS and HSR were in disagreement in four of 17 cases (24%) (Figure 5B). Out of the 17 discordant cases, iFR agreed with CFVR in 16 cases (94%), whilst FFR agreed with CFVR in one case (6%) (Figure 5B).
Discussion
The present study constitutes the first head-to-head comparison of BSR, iFR, and FFR against an independent reference standard of stenosis-induced myocardial ischaemia. Our results show that basal, vasodilator-free, physiological indices BSR and iFR are equivalent to FFR in identifying those stenoses that cause myocardial ischaemia. This is true for both ROC curve-derived ischaemic, as well as FFR 0.80-equivalent clinical cut-off values. When dichotomous classification of BSR or iFR disagreed with FFR, FFR agreed with the reference standard in half of the cases, while BSR and iFR agreed with the reference standard in the other half. These results indicate that both BSR and iFR may be considered accurate indices of functional stenosis severity, and worthy of further clinical investigation as possible vasodilator-free alternatives to FFR.
DIAGNOSTIC PERFORMANCE OF ADENOSINE-FREE INDICES COMPARED WITH FFR
The finding that BSR and iFR have equivalent diagnostic performance compared with FFR to identify ischaemia-generating coronary stenoses is consistent with recent publications14,16,24. It is important to emphasise that, at a difference with BSR13, iFR has never been evaluated against an independent reference standard14-16,26. In this regard, our observations provide complementary information to that generated in previous studies using FFR as the reference standard to evaluate the performance of iFR, and add to the accumulating evidence that the functional severity of a coronary stenosis may be adequately assessed without the use of pharmacologically induced vasodilation.
Consistent with the findings in the initial validation study13, BSR was found to have equivalent diagnostic accuracy for the identification of ischaemia-generating coronary stenoses at its ischaemic cut-off value of 0.66 mmHg cm−1 sec compared with FFR at its 0.75 ischaemic cut-off value. Moreover, we found that a (ROC curve-derived) clinical BSR cut-off value of 0.47 mmHg cm−1 sec yields a diagnostic performance equivalent to that of FFR at the clinical 0.80 cut-off. Similarly, the diagnostic performance of iFR at its optimal ischaemic cut-off value was found to equal the diagnostic performance of FFR at its ischaemic cut point. Moreover, the previously established clinical iFR cut-off value of 0.90 was equivalent to the clinically established FFR cut-off of 0.80, supporting the findings in a recent large patient-level pooled analysis of studies comparing iFR with FFR24. Additionally, the discriminative value of iFR was not improved by the administration of adenosine (Figure 2B, Table 5) even though iFRa was numerically lower than both iFR and FFR (Table 2), thereby confirming the findings in a recent smaller study15.
Apart from the equivalence of both BSR and iFR to FFR, a novel finding in the present study is that BSR and iFR are also similar to each other in terms of discriminative value, as well as dichotomous classification agreement with the reference standard at both the ischaemic and the clinical cut-off values. These results indicate that BSR and iFR may both provide justifiable vasodilator-free alternatives for FFR. Nonetheless, at a difference with iFR, BSR provided incremental discriminative power over resting Pd/Pa in both the full cohort as well as the iFR cohort, suggesting a potential diagnostic advantage of combining coronary pressure and flow under resting conditions.
Discordance between BSR or iFR and FFR
A strong limitation of prior comparisons of dichotomous physiological diagnostic tests without an independent reference standard, like iFR and FFR, is that discordance in classification agreement is influenced by the frequency distribution of test values, as discussed by Petraco et al14. Moreover, almost all have used a single test as standard of reference. Such an approach is by definition limited by the limitations of the reference standard itself. By combining only true positives from two different and concurrent tests, the approach used in our study selected as a standard of reference only those cases in which inducible ischaemia was present and most likely due to the epicardial stenosis. In that regard, being a head-to-head comparison with an independent reference standard of true stenosis-induced myocardial ischaemia, our study provided an unique opportunity to clarify classification mismatches between adenosine-free indices and FFR. We observed that, although both adenosine-free indices were diagnostically as efficient as FFR, discordance with FFR was not infrequent: at their ischaemic cut-offs, discordance with FFR occurred in 18% and 19% of cases for BSR and iFR, respectively. In those discordant cases, FFR was found to be correct in only half of cases, and adenosine-free indices were found to be correct in the other half of cases. This observation is of key importance, since FFR has been proposed as an invasive gold standard for the detection of myocardial ischaemia26, and has served as the comparator for other physiological indices of stenosis severity. Given the findings in the present study, future validation studies of novel physiological indices should perhaps be limited to using independent reference standards, instead of FFR, to establish their diagnostic performance.
ISCHAEMIC VERSUS CLINICAL CUT-OFFS
In the present study, we evaluated the diagnostic performance of BSR, iFR, and FFR using both their ischaemic as well as their clinical cut-off values. We found that the diagnostic performance of both BSR and iFR was equivalent to FFR, regardless of the type of cut-off used. In contrast with the hypothesis that governed the transition from the ischaemia-derived 0.75 FFR cut-off value to a 0.80 cut point, which aimed to minimise the number of ischaemic stenoses inadvertently left untreated5, we observed that this increase in cut-off value resulted in an increase in the false positive rate from 9.4% (28 out of 299) to 20.4% (61 out of 299) of coronary stenoses, an 11% increase (33 out of 299). These 33 stenoses, that fall in the 0.75-0.80 FFR grey zone, were all identified as non-ischaemic by the combined reference standard, with a mean CFVR of 2.4, and mean HSR of 0.53 mmHg cm−1 sec, suggesting no significant impediment to coronary flow. In contrast, there was only a 0.7% (two out of 299) reduction in false negative results when using the clinical 0.80 cut point. A similar increase in false-positives was also observed using either BSR or iFR when moving from ischaemic to clinical cut-off values. Therefore, although the use of clinical cut-off values for revascularisation decision making has the theoretical advantage of being cautious, and reducing the probability of missing ischaemia-generating stenoses, this advantage comes at the expense of treating stenoses that do not unequivocally cause myocardial ischaemia. The clinical significance of this step away from an ischaemia-derived cut point to a clinical cut point should therefore not be underestimated and may in part help explain the high number of stenoses that were classified as ischaemia-generating in FAME II, but did not require revascularisation within the first year of follow-up2.
WHY ARE BSR AND iFR ABLE TO DETERMINE FUNCTIONAL STENOSIS SIGNIFICANCE?
It is frequently assumed that vasodilation is a requisite for physiological stenosis evaluation, as it would unmask trans-stenotic gradients that are not present during resting conditions. This assumption implies that basal parameters are prone to inaccuracies, and in particular to false negative stenosis classification. However, although pressure gradients and velocities during basal conditions are smaller, BSR was found to be equivalent to adenosine-mediated FFR, both in terms of discriminative value and the number of false-negative stenosis classifications. Similar findings related to the iFR to FFR comparison. These findings suggest that, contrary to current assumptions25,27, the absence of vasodilator-induced hyperaemia does not impair discriminatory power, and highlights the difference between indices in how they discriminate between stenosis severities. While FFR relies on the overriding effect of adenosine on coronary autoregulation to increase flow, and thereby unmask the haemodynamic repercussions of the stenosis, discrimination of stenosis severity by BSR is facilitated by high-fidelity measurement of both pressure and flow velocity13. Discrimination by iFR is facilitated by measuring the pressure gradient during a specific period in diastole, when flow velocity is intrinsically higher16.
While a uniform effect of adenosine is a requisite for the concept of FFR, its effect actually varies between patients28,29 and varies inversely with epicardial stenosis severity15,30. Hence, it is possible to speculate that in some cases basal measurements may even be beneficial, and may help resolve the well-established discordance between hyperaemic pressure and flow indices28. This is perhaps most evident in the iFR-FFR discordant group, where iFR closely agreed with CFVR in 94% of cases (Figure 5B), suggesting that iFR measured under basal conditions is strongly predictive of the vasodilator reserve of the coronary artery under investigation15.
Clinical implications
The present study adds to the premise that the assessment of the functional severity of a coronary artery stenosis can be accurately performed during basal conditions, without concerns about an inadvertent increase in stenoses falsely deferred. Although FFR has been adopted by physiology-minded catheterisation laboratories, its worldwide adoption remains low7,8, in which the requisite to administer potent vasodilators9-11, such as adenosine, is considered an important denominator12. Not only may the use of adenosine be associated with insurmountable side effects, or may even be contraindicated such as in patients with asthma or COPD, but additional uncertainty exists regarding the ability of adenosine to induce a “true maximal hyperaemic state”17,31, which is critical in the concept of FFR9,12,17. Some studies have indicated that maximal hyperaemia induced by intravenous adenosine infusion may be enhanced by concomitant administration of other vasodilator drugs, such as alpha-blockers and angiotensin-converting enzyme inhibitors32-34. Hence, a vasodilator-free approach may provide an opportunity to facilitate a more widespread adoption of physiologically guided revascularisation in clinical practice, and circumvent any uncertainties and ambiguities associated with the dosing and administration of adenosine.
Limitations
The study population under investigation for iFR resembles only a sample from the source population. However, clinical characteristics of the iFR cohort mirror those of the source population, and may therefore be considered a representative study sample. Furthermore, this is the largest study to date to assess iFR, BSR, and FFR with an independent reference standard.
It should be noted that there is no true gold standard for the presence of inducible myocardial ischaemia. A perfusion defect observed during myocardial scintigraphy is no direct proof of the presence of myocardial ischaemia, but particularly of the presence of marked perfusion inequality in the myocardium. Despite this limitation, stress myocardial perfusion scintigraphy is considered a well-established stress imaging technique, recommended for the identification of inducible myocardial ischaemia35,36. Importantly, the absence of a true gold standard ischaemia test is an inherent limitation to the establishment of any new physiological index aiming to identify stenosis-related reversible myocardial ischaemia. In the present study, we therefore used a combined reference standard of MPS and HSR, allowing identification of perfusion maldistribution by means of MPS, indicative for the presence of inducible myocardial ischaemia, which could be most accurately related to the stenosis of interest by means of HSR.
With the currently available armamentarium, assessment of intracoronary blood flow velocity is a technique that is subject to technical failures, and accurate evaluation of coronary blood flow velocity is dependent on the experience of the cardiologist. All coronary flow velocity measurements in this study were performed by operators with ample experience in intracoronary flow velocity measurements. Nonetheless, widespread adoption of this technique is dependent on currently ongoing improvements in the available armamentarium to facilitate the feasibility of the stenosis resistance index as a diagnostic tool in daily clinical practice.
Finally, in the present study, hyperaemia was induced by means of an intracoronary bolus of adenosine. Although there is an ongoing debate regarding the dose of adenosine needed to induce true maximal hyperaemia, the dose used in the present study has been extensively validated for its relationship with non-invasively assessed myocardial ischaemia17, is known to result in FFR values equal to those induced by intravenous administration of adenosine17, and is moreover associated with equivalent clinical benefit of FFR-guided revascularisation37,38.
Conclusion
Both BSR and iFR have equivalent diagnostic accuracy compared with FFR for the identification of ischaemia-generating coronary stenoses. BSR and iFR may therefore be considered worthy of further exploration in terms of their clinical value as diagnostic tools for functional coronary stenosis severity assessment.
| Impact on daily practice Although FFR has been adopted by physiology-minded catheterisation laboratories, its worldwide adoption remains low. A vasodilator-free approach, alleviating the need for potent vasodilators, may provide an opportunity to facilitate a more widespread adoption of physiologically guided coronary revascularisation in clinical practice, and circumvent any uncertainties and ambiguities associated with the dosing and administration of adenosine. The present study adds to the accumulating evidence that functional stenosis severity can be accurately assessed under resting conditions. |
Acknowledgements
The authors gratefully acknowledge the nursing staff of the cardiac catheterisation laboratory of the Academic Medical Center, Amsterdam, The Netherlands (Head: M.G.H. Meesterman) for their skilled assistance.
Funding
This study was funded in part by the European Community’s Seventh Framework Programme (FP7/2007-2013) under grant agreement no. 224495 (euHeart project), and by grants from the Dutch Heart Foundation (2006B186, 2000.090, and D96.020). S. Sen (G1000357), and S. Nijjer (G1100443) are Medical Research Council fellows. J. Davies (FS/05/006) and R. Petraco (FS/11/46/28861) are British Heart Foundation fellows.
Conflict of interest statement
J. Davies holds patents pertaining to the instantaneous wave-free ratio (iFR) technology. All the other authors have no conflicts of interest to declare.

