
Abstract
Aims: The association between fractional flow reserve (FFR) and dobutamine stress echocardiography (DSE) in real-world stable angina patients is scant and controversial whereas no such comparison exists with instantaneous wave-free ratio (iFR). The current retrospective study aimed to investigate the associations among these modalities in patients with stable coronary artery disease (CAD) and intermediate coronary lesions.
Methods and results: We studied 62 consecutive stable angina patients who underwent DSE and subsequently coronary angiography with FFR (in all 62) and iFR (in 46/62 patients) assessment of intermediate single-vessel lesions between 2014 and 2015. Using receiver operating characteristic (ROC) curves we sought to identify the optimal FFR and iFR cut-off points with the highest discriminative power to predict the DSE result. The kappa coefficient was used to assess the agreement between FFR, iFR and DSE. The mean age of the study cohort was 63.5±12 years and 35 (56.5%) were males. Thirteen (21%) lesions were adjudicated as causing reversible ischaemia on DSE. Using ROC (FFR predicting DSE result), the area under the curve was 0.952 (95% CI: 0.902 to 1), whereas for iFR it was 0.743 (95% CI: 0.560 to 0.927), pAUC comparison=0.03. The optimal FFR cut-off point predicting positive DSE was 0.80. There was strong agreement between DSE and FFR (kappa 0.682, p<0.001). There was only modest agreement between iFR and DSE (kappa 0.258, p=0.068) using a cut-off value of 0.9.
Conclusions: In patients referred for evaluation of stable CAD, there was good agreement between DSE and FFR (87%) but less so with iFR (71.7%).
Abbreviations
DSE: dobutamine stress echocardiography
FFR: fractional flow reserve
iFR: instantaneous wave-free ratio
MI: myocardial infarction
ROC: receiver operating characteristic
Introduction
The safety and feasibility of dobutamine stress echocardiography (DSE) in patients following myocardial infarction (MI) was first demonstrated by Berthe et al1 in the early 1980s. Pierard et al2 validated this method against positron emission tomography in 17 patients while Picano et al revealed that DSE is safe and can be conclusive without any complications in 88% of cases3. The prognostic value of DSE was initially shown in a cohort of 778 post-MI patients4 and established in a meta-analysis of 5,946 patients5, demonstrating a negative predictive value (NPV) for MI and cardiac death of 98.4% over the 33 months of follow-up.
In the early 1990s Pijls et al6 introduced the invasive coronary fractional flow reserve (FFR), adding a functional assessment to the anatomy-dominated world of coronary intervention. In a seminal paper7, FFR was compared to a combination of several functional tests, among them DSE, and demonstrated satisfactory diagnostic accuracy in predicting a positive functional test using a cut-off of 0.75. The prognostic significance of FFR has been demonstrated in large randomised trials8-10 using cut-offs of between 0.75 and 0.8 when defining a positive result. In 2012, the instantaneous wave-free ratio (iFR) non-hyperaemic index was introduced11. It was claimed to be able to predict a positive FFR result accurately (r=0.9, p<0.001) with a receiver operating characteristic (ROC) area under the curve (AUC) of 0.93, at FFR <0.8.
In a recent paper by Wu et al12, 67 vessels with 50% to 80% diameter stenosis by quantitative coronary angiography (QCA) in 58 consecutive patients were examined with FFR and real-time myocardial contrast echocardiography (RTMCE). Even though 17/18 stenoses that were FFR positive had abnormal capillary blood flow (CBF) during RTMCE, 28/49 stenoses (57%) that were FFR negative also had abnormal CBF in the corresponding coronary artery territory during stress echocardiography. To our knowledge, to date no studies have compared both FFR and iFR to contrast-enhanced DSE.
In this study we aimed to identify the agreement between FFR, iFR and contrast-enhanced DSE using second-generation contrast agents in real-world stable angina patients with single intermediate coronary artery lesions. Furthermore, we aimed to identify the optimal FFR and iFR cut-off values that predict the presence of reversible ischaemia during DSE in such patients.
Methods
Two hundred and seventy-eight patients with stable angina underwent DSE and subsequently coronary angiography at Imperial NHS Trust Hospitals (Hammersmith, St. Mary’s and Charing Cross) between 2014 and 2015. Patients with negative DSE underwent coronary angiography if their symptoms were typical. Eighty-three of them had lesions of intermediate severity and underwent invasive FFR. Interoperator variability for contrast DSE was excellent with a kappa of 0.92. Patients with inconclusive DSE imaging, previous CABG, multivessel disease, full thickness infarct of the culprit artery, sequential lesions, diffuse disease or significant valvular disease were excluded. In particular, after excluding five patients who had inconclusive DSE, 10 who had previous CABG, multivessel disease, tandem lesions in a single vessel or distal diffuse disease and six who had significant valvular disease, the final patient cohort consisted of 62 patients. In 16 of these patients iFR measurements were not performed due to operator preference. These patients had single-vessel, single lesion disease with no prior MI related to the target vessel. The hospital ethics committee approved this retrospective study and all participants signed informed consent for relevant procedures.
DOBUTAMINE STRESS ECHO
All patients underwent contrast DSE following the protocol suggested by the European Association of Echocardiography recommendations. Details are described in the Supplementary Appendix 1.
PRESSURE WIRE STUDIES
Coronary angiography and pressure-flow assessments of coronary stenoses were performed using conventional approaches within three months from DSE13. No changes in ischaemic symptoms or medical therapy occurred in any of the patients included in the study. Details of the procedures are described in the Supplementary Appendix 2.
QUANTITATIVE CORONARY ANGIOGRAPHY
QCA parameters (diameter stenosis [DS] %, minimal lumen diameter [MLD] mm, minimal lumen area [MLA] mm2, area stenosis [AS] %, reference vessel diameter [RVD] mm, and reference vessel area [RVA] mm2) were calculated using dedicated workstations (CAAS II; Pie Medical, Maastricht, the Netherlands).
STATISTICAL ANALYSIS
Results are presented as means±SD, medians (interquartile range) or percentages. In order to identify the optimal cut-off point for maximum diagnostic accuracy between FFR and DSE, ROC curves and the Youden’s J statistic were used. We used sensitivity, specificity, positive and negative predictive values and the kappa coefficient to assess the agreement between FFR, iFR and DSE. Differences between areas under the curve (AUC) were compared using the Hanley and McNeil test14.
Results
A total of 62 patients with single intermediate angiographic lesions (50-80% visually estimated maximum lumen narrowing) were assessed over the two-year period. Mean age was 63.5±12 years and 35 (56.5%) were males. Baseline demographics of the study population are presented in Table 1 and Table 2. The LAD was assessed in 53 cases (85.5%), the LCx in two (3.2%) and the RCA in seven (11.3%). The mean DS was 48.5±8.3% whereas the MLD was 1.44±0.32 mm (Table 1).
Of the 46 patients who also underwent iFR, 40 (87%) had their LAD assessed, two (4.3%) their LCx and four (8.7%) the RCA. iFR evaluation always preceded FFR.
DOBUTAMINE STRESS ECHO AS THE GOLD STANDARD
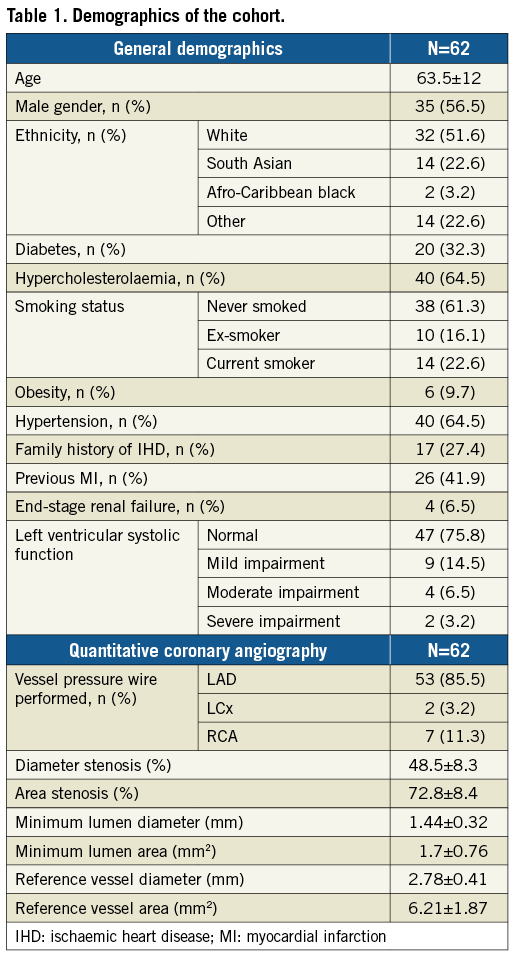
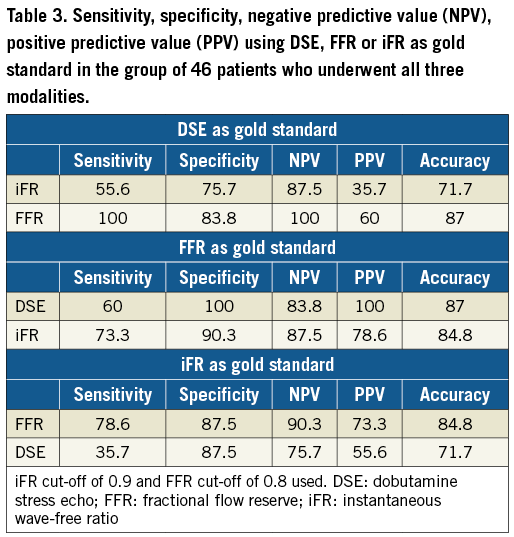
Thirteen (21%) of these lesions were adjudicated as causing reversible ischaemia during DSE. The AUC for FFR predicting a DSE result was 0.952 (95% CI: 0.902 to 1.00), p<0.001 (Figure 1A). The optimal FFR cut-off point (≥) to predict a negative DSE result was 0.8. Using this cut-off, 21 (33.9%) of these lesions were rendered “flow-limiting” (Figure 1B). There was a strong agreement between the two modalities (kappa 0.682, p<0.001). Using DSE as the gold standard, FFR (with a cut-off point of 0.8) had a sensitivity of 100% and a specificity of 83.7%. The positive predictive value was 61.9%, whereas the negative predictive value was 100%. Diagnostic agreement was achieved in 87% of patients (54/62).
Figure 1. Agreement between FFR and DSE. A) ROC curve demonstrating good correlation of FFR with DSE. B) Scatter plot demonstrating the agreement between FFR using the 0.8 cut-off and DSE across the range of anatomic lesions.
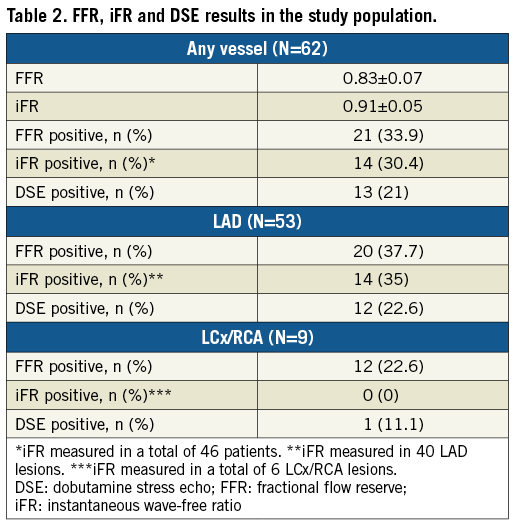
In the 46 patients who had both FFR and iFR measurements, the AUC for iFR predicting a positive DSE result was 0.743 (95% CI: 0.560 to 0.927), p=0.025 (Figure 2A). The optimal iFR cut-off to predict a DSE result was 0.90. Using this cut-off value, 14 (30.4%) lesions were rendered haemodynamically significant (Figure 2B). There was a modest agreement between iFR and DSE (kappa 0.258, p=0.068). Using DSE as the gold standard, iFR (with a cut-off value of 0.9) had a sensitivity of 55.6%, a specificity of 75.7%, a positive predictive value of 35.7% and a negative predictive value of 87.5%. Diagnostic agreement would have been achieved in 33/46 (71.7%).
Figure 2. Agreement between iFR and DSE. A) ROC curve demonstrating the modest correlation of iFR with DSE. B) Scatter plot demonstrating the agreement between iFR using the 0.90 cut-off and DSE across the range of anatomic lesions.
Amongst the 46 patients who underwent both FFR and iFR measurements, the AUC, for pressure wire measurements using DSE as the gold standard, was significantly higher for FFR (0.935, 95% CI: 0.864 to 1.00) compared to iFR (0.743, 95% CI: 0.560 to 0.927); AUC difference 0.192 (SE difference 0.09, p=0.03) (Figure 3).
A summary of sensitivity, specificity, NPV, PPV using DSE, FFR and iFR as gold standards is shown in Table 3.
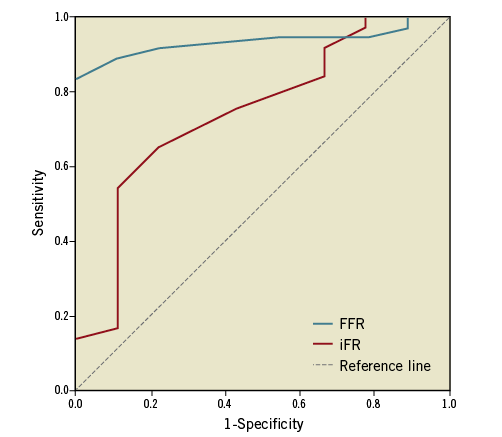
Figure 3. ROC curves for FFR and iFR using DSE as the gold standard.
Discussion
In this study we demonstrated a good correlation between contrast-enhanced DSE using second-generation contrast agents and invasive FFR measurements but a worse correlation with iFR in predicting reversible myocardial ischaemia in consecutive stable angina patients with intermediate coronary artery lesions. Using ROC curves, we identified that the optimal FFR cut-off value to predict the DSE result was 0.8, which coincides with the currently used cut-off in clinical practice.
In a seminal paper by Pijls et al7, FFR was compared to exercise testing, thallium scintigraphy, DSE and quantitative arteriography in 45 consecutive patients with moderate coronary stenosis and chest pain, prospectively testing the earlier cut-off value of 0.7515 to a true gold standard based upon the combination of the three non-invasive tests, all performed within 24 hours from the FFR measurement. Using a multi-testing sequential Bayesian approach, the sensitivity of FFR in the identification of reversible ischaemia was 88%, the specificity 100%, the positive predictive value 100%, and the negative predictive value 88%. In another early study of 75 stable angina patients16, the degree of dobutamine-induced dyssynergy correlated well with FFR using a cut-off of 0.75 (r=0.77). Despite the excellent PPV of 98%, a very low NPV (61%) was observed using this cut-off. Based on this cut-off, the DEFER study6 did not show any significant difference in patient outcomes with FFR ≥0.75 who underwent percutaneous coronary intervention compared to those treated medically. If a value of 0.80 had been used in the study by Piljs7, the sensitivity would have been 100% and the specificity 92%, figures pretty similar to the ones reported in our study. In order to avoid not treating an ischaemic lesion rather than treating a non-ischaemic lesion, in the FAME8 and FAME 217 trials a cut-off value of 0.80 was used, demonstrating the prognostic benefits of FFR-guided revascularisation. In a more recent retrospective study, in an attempt to justify the use of the 0.8 cut-off in clinical practice, Adjedj et al18 investigated the outcomes of patients with single lesions in the so-called “grey zone” of FFR 0.76 to 0.80 who were either revascularised or treated with medical therapy alone. Of interest, even though there was no significant difference in overall major adverse cardiac events (MACE), there was a trend for higher mortality (p=0.059) and the combined outcome of MI/death (p=0.06) in the medical therapy group.
In the current study without presumptions, we have shown that the optimal FFR cut-off value to predict reversible myocardial ischaemia during DSE was 0.8. Using this cut-off, the negative predictive value was 100%, rendering it safe practice to treat patients with FFR values above 0.8 conservatively. In an early study by de Bruyne et al19, a close correlation was found between relative flow reserve obtained by positron emission tomography (PET) and myocardial FFR (r=0.87), once again proving the validity of FFR against robust non-invasive modalities. In a recent meta-analysis by Danad et al20, the sensitivity of DSE in predicting FFR was 75% (compared to 61.9% in the current study) and the specificity only 75% (compared to 100%). The discrepancies in the sensitivity and specificity figures can be attributed to differences in the population studied and the fact that we used contrast DSE rather than plain DSE. In the study by Jung et al21, there was a significant improvement in sensitivity (for predicting FFR) from 48 to 83% when using contrast compared to plain imaging. In a small study of just 21 patients, Jimenez-Navarro et al22 demonstrated only a moderate correlation of FFR with DSE with a kappa value of 0.51. The improvements of spatial resolution and image quality in modern cardiac ultrasound systems along with standardisation of the DSE examination23 and the routine use of second-generation contrast agents may explain the better diagnostic accuracy observed in our study. In the COMPRESS trial24, contrast echo DSE and single photon emission computed tomography (SPECT) were compared to FFR in 48 patients, 41 of whom had multivessel disease. The sensitivity of DSE in this patient substrate was similar to that in our study at 67% (61.9% in our study), whereas specificity was much lower at 77% (100%). The reduced specificity is partially explained by the different study population and design. Recently, Wu et al12, putting a new spin on non-invasive test vs. pressure wire comparisons, showed that almost half of patients with negative pressure wire studies might suffer from microvascular disease, as evidenced by reductions in CBF. This suggests that the presence of severe microvascular disease can be missed by FFR, hence a separate methodology to assess the microvasculature is mandatory.
Recently, iFR has been validated against FFR25-28 with ROC curves against FFR ranging from 0.8126 to 0.927, but no comparison with DSE exists as yet. A comparison of iFR and PET perfusion imaging revealed a ROC of 0.86 for iFR, which was similar to the one for FFR at 0.85 (p=0.71). In our study, the ROC curve between iFR and FFR was also within the aforementioned range at 0.85329. Most operators in our institution would not perform FFR routinely in lesions with iFR or Pd/Pa over 0.95 or lower than 0.8, hence lesions with much higher agreement between iFR and FFR have been excluded. This selection bias seen in our study is a reflection of the real-world experience and hence much more informative compared to studies which include cases at the extreme ranges of iFR and FFR. Recently, two large non-inferiority randomised trials compared the prognostic value of iFR versus FFR30,31, establishing that coronary revascularisation guided by iFR is non-inferior to revascularisation guided by FFR with respect to risk of MACE at one year. Interestingly, despite similar one-year MACE, in both studies there were significantly more haemodynamically significant lesions in the FFR group30,31, leading to more revascularisation procedures30. These two large iFR studies suggest that, by using the 0.8 FFR cut-off, physicians may be overtreating patients who could potentially do just as well with medical therapy. The modest agreement between iFR and DSE shown in the present study highlights the difficulties in achieving an optimal revascularisation cut-off consensus amongst different modalities and the need for larger outcome trials that incorporate non-invasive and pressure wire studies in their design.
Limitations
Limitations of the current study include its retrospective nature and the presence of a small time lag, albeit <3 months, between pressure wire studies and DSE. In the majority of cases the LAD was assessed, hence results should be viewed with caution when assessing non-LAD arteries. Another factor that should be taken into account is the potential for selection bias, as only patients with truly intermediate stenosis would have gone on to invasive pressure wire assessments. Nevertheless, it is the largest study yet comparing FFR with contrast-enhanced DSE using contemporary new-generation echocardiographic platforms with the routine use of second-generation contrast agents and pressure wire devices, and the first to use ROC curves to identify optimal FFR and iFR cut-offs to predict a DSE result. Furthermore, this is the first study to compare iFR with DSE directly. Future, multicentre studies are required to confirm our findings in large patient cohorts.
Conclusions
The current study shows that contrast-enhanced, state-of-the-art DSE correlates strongly with FFR but less so with iFR in real-world patients with single moderate coronary artery lesions. This highlights that contrast DSE can be used as a good gatekeeper, keeping patients with atypical symptoms and negative DSE studies away from invasive procedures. However, when positive, FFR is more likely to be in line with the DSE results compared to iFR.
|
Impact on daily practice In real-world patients, contrast-enhanced, state-of-the-art DSE is a good gatekeeper for invasive procedures. When DSE is positive, FFR is more likely to agree with DSE compared to iFR. |
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplementary data
Supplementary Appendix 1. Dobutamine stress echo.
Supplementary Appendix 2. Pressure wire studies.
To read the full content of this article, please download the PDF.

