Abstract
Invasive coronary physiology to select patients for coronary revascularisation has become established in contemporary guidelines for the management of stable coronary artery disease. Compared to revascularisation based on angiography alone, the use of coronary physiology has been shown to improve clinical outcomes and cost efficiency. However, recent data from randomised controlled trials have cast doubt upon the value of ischaemia testing to select patients for revascularisation. Importantly, 20-40% of patients have persistence or recurrence of angina after angiographically successful percutaneous coronary intervention (PCI). This state-of-the-art review is focused on the transitioning role of invasive coronary physiology from its use as a dichotomous test for ischaemia with fixed cut-points, towards its utility for real-time guidance of PCI to optimise physiological results. We summarise the contemporary evidence base for ischaemia testing in stable coronary artery disease, examine emerging indices which allow advanced physiological guidance of PCI, and discuss the rationale and evidence base for post-PCI physiological assessments to assess the success of revascularisation.
Physiological justification of PCI
INTRODUCTION
Contemporary guidelines suggest that the principal remit of percutaneous coronary intervention (PCI) in stable coronary artery disease (CAD) is to treat symptoms refractory to optimal medical therapy (OMT)1. Recent data from the largest trial studying the impact of an early invasive strategy on hard outcomes in stable CAD support these recommendations2. Since we attribute symptoms not to the anatomic stenoses but to their association with myocardial perfusion, it intuitively follows that, to treat angina optimally, improvement of physiological, rather than anatomical characteristics of a diseased vessel should be the principal goal. Ischaemia-guided revascularisation with invasive coronary physiology has become a central component of modern-day evidence-based treatment algorithms for the management of stable CAD1. By offering real-time assessment of the ischaemic significance of epicardial stenoses, invasive coronary physiology identifies patients who can benefit from revascularisation by bridging the gap between anatomy, physiology and symptoms. However, recent placebo-controlled data have challenged this paradigm and it has become apparent that the link between ischaemia and symptoms may be more complex3.
Physiological assessment allows vessel- and lesion-specific assessment for ischaemia. Therefore, in optimising post-PCI physiology, it may be possible to improve outcomes specific to that vessel or lesion. However, it must be remembered that cardiovascular events also occur from angiographically and physiologically non-significant lesions, so, even with the best physiological results, secondary prevention with optimum medical therapy remains essential4.
In this state-of-the-art review, we report the contemporary evidence base for revascularisation based on invasive physiology, the evolving role of physiology to guide PCI strategy, and the potential value of post-PCI physiological assessments. More broadly, we discuss the current role of ischaemia testing in the diagnosis and treatment of stable CAD and the implications for the future of coronary physiology.
CORONARY PHYSIOLOGY INDICES AND CLINICAL OUTCOME DATA
FRACTIONAL FLOW RESERVE
Summary characteristics and trial designs for landmark fractional flow reserve (FFR) trials are presented in Table 1.
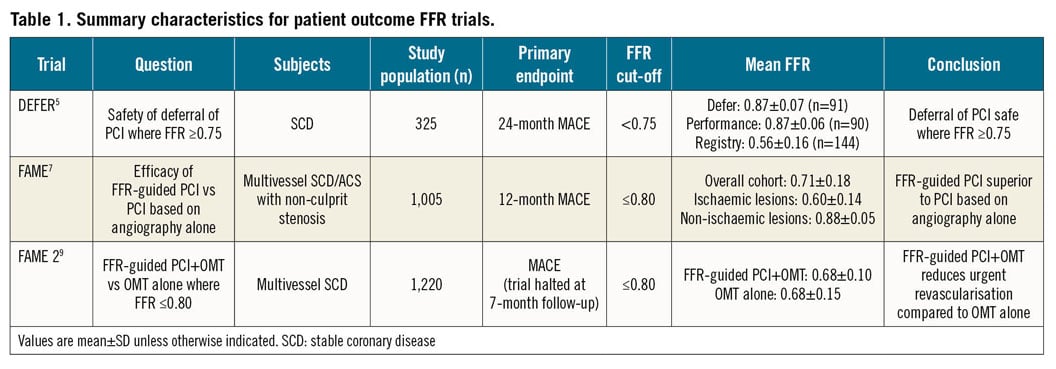
In an era where interventionalists believed that anatomy was the gold standard and that treatment of moderate lesions was “preventative”, the early FFR trials truly challenged dogma. The DEFER (Deferral Versus Performance of PTCA in Patients Without Documented Ischemia) trial demonstrated that, even in the presence of a significant single vessel anatomical stenosis, there were patients in whom it was safer to opt for conservative treatment5. The trial showed that, when FFR was ≥0.75, optimum medical therapy alone was equally safe to revascularisation with PCI and optimum medical therapy5. Importantly, it also showed that PCI did not lead to eradication of hard clinical outcomes because the reference group with FFR <0.75, in whom PCI was performed albeit in the bare metal stent era, had the worst outcomes among the three groups6.
Once DEFER had shown that it was safe to leave non-physiologically significant lesions alone, the next step was to investigate whether FFR could be used to guide whom to treat with the larger FAME (Fractional Flow Reserve versus Angiography for Multivessel Evaluation) trial. The investigators this time chose a higher “clinical” FFR cut-point of 0.80 to ensure a safety margin for revascularisation at a time when the accepted belief was, “If you see it, you must treat it”. They randomly assigned patients with multivessel CAD to revascularisation based on angiography alone or to revascularisation for any vessel with FFR ≤0.807. At 12-month follow-up, there was a significantly lower incidence of the primary composite endpoint of death, non-fatal myocardial infarction (MI) and repeat revascularisation in the FFR group, indicating superiority over angiography alone. Importantly, there was no difference in symptom improvement by Canadian Cardiovascular Society angina grade between the FFR- and angiography-guided groups7.
The DEFER and FAME trials served to prove that, in stable CAD, PCI for stenoses deemed to be “non-ischaemic” according to FFR thresholds was less effective than conservative management, and possibly even harmful5,7. These landmark trials also importantly questioned the role of anatomically guided treatment and showed that reduction in number and length of stents was clinically advantageous in terms of both clinical outcomes and cost-effectiveness8.
The FAME 2 (Fractional Flow Reserve guided PCI versus Medical Therapy in Stable Coronary Disease) trial was designed to answer whether a strategy of PCI in addition to medical therapy was superior to medical therapy alone for patients with significant ischaemia, defined as an FFR ≤0.80 in at least one vessel9. This unblinded trial was terminated early on ethical grounds because of a marked reduction in the rate of urgent revascularisation in the PCI+OMT group. The large majority of these unplanned revascularisation events were not associated with electrocardiographic (ECG) changes or biomarker elevation and, as a result, the endpoint was vulnerable to bias10. There was no significant difference in the incidence of all-cause mortality, cardiac death and MI, although there was a significant reduction in spontaneous MI among patients undergoing PCI at longer-term follow-up11,12.
INSTANTANEOUS WAVE-FREE RATIO
Despite evidence that the use of FFR was clinically and economically efficacious, global adoption of FFR remained limited13. Added time, cost, and lack of familiarity with the techniques may have been barriers to its routine use14. A resting index, the instantaneous wave-free ratio (iFR), that did not require the induction of pharmacological hyperaemia, was developed to tackle some of these issues. The diagnostic accuracy of iFR to identify FFR-positive lesions was good and, when a third comparator was used as a reference standard, no significant differences were observed between FFR and iFR for their accuracy in identification of ischaemic stenoses15. Randomised controlled trials that were essential for the clinical validation of iFR are summarised in Table 2.
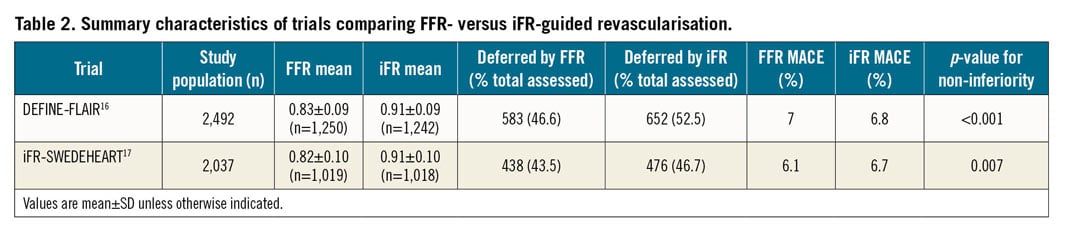
DEFINE-FLAIR (Functional Lesion Assessment of Intermediate Stenosis to Guide Revascularisation)16 and iFR-SWEDEHEART (Evaluation of iFR vs FFR in Stable Angina or Acute Coronary Syndrome)17 were non-inferiority trials designed to test for equivalence between iFR- and FFR-guided revascularisation. The trials utilised identical cut-points for PCI (iFR ≤0.89 and FFR ≤0.80) with a harmonised primary endpoint of 12-month incidence of all-cause mortality, non-fatal MI and unplanned revascularisation.
With concordant results across the two trials, iFR-guided revascularisation was found to be non-inferior to an FFR-guided strategy. In a pooled patient-level analysis of the DEFINE-FLAIR and iFR-SWEDEHEART trials, it was found that significantly more patients were deferred in the iFR arm, with no impact on clinical outcomes18.
Treatment reclassification occurs in 22-48% of studied populations when an FFR measurement is used in addition to the coronary angiogram to guide therapy in stable CAD19. In a substudy from iFR-SWEDEHEART, the use of iFR or FFR reclassified the initial angiogram-based treatment decision in approximately 40% of cases. The majority of these patients were reclassified from a strategy of revascularisation with PCI to conservative therapy with OMT20. No significant differences were observed between the reclassified iFR and FFR populations.
NOVEL NON-HYPERAEMIC PRESSURE RATIOS
Following the successful introduction and adoption of iFR in clinical practice, a number of alternative non-hyperaemic pressure ratios (NHPRs) have emerged in recent years. The commonality among these indices is a sub-cycle pressure measurement of the ratio of distal coronary to proximal aortic pressure (Pd/Pa). As these measurements are made independently of hyperaemia, they share in the principal advantage of iFR, namely, the lack of requirement of a vasodilator agent such as adenosine. The principal point of difference between each NHPR lies in the period of the cardiac cycle in which the Pd/Pa ratio is measured, the so-called “sampling window”. The advantages and disadvantages of NHPRs in comparison to hyperaemic indices are presented in Table 3.
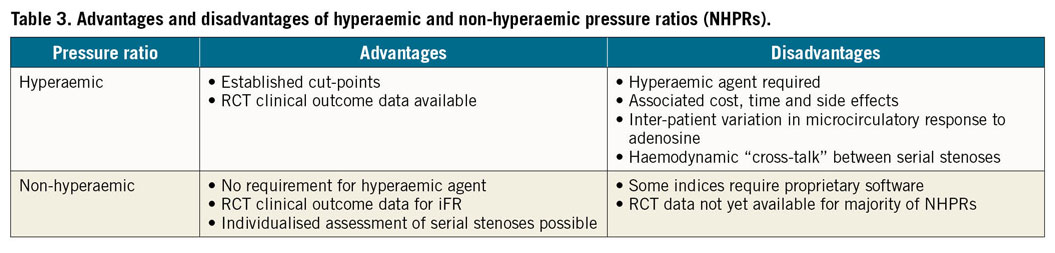
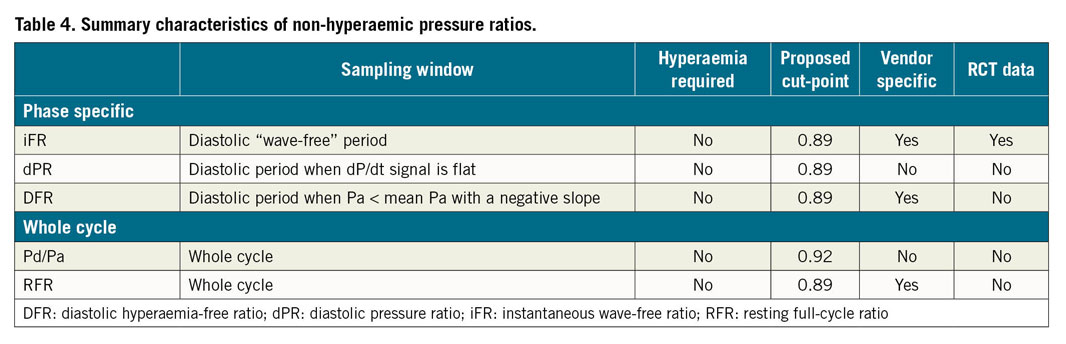
Several NHPRs have been shown to be closely related to iFR, both numerically and in terms of the accuracy of their classification when compared to a “gold standard” of FFR21. However, some indices, such as whole cycle Pd/Pa, may be more vulnerable to pressure wire drift causing increased diagnostic reclassification compared to iFR and FFR22. Despite evidence suggesting diagnostic equivalence of NHPRs, there have been no prospective randomised clinical outcome trials of any of these newer NHPRs. Characteristics of the most commonly used NHPRs are presented in Table 4.
DEFINING THRESHOLDS
Defining a cut-point for treatment is a necessity when designing a clinical trial assessing the use of a measure of ischaemia. Trial investigators must follow strict algorithms in order to reduce the incidence of protocol deviation. Cut-points also aid statistical analysis and interpretation of the results. However, any measure of invasive physiology is continuous by its biological nature23. There can be no binary cut-point at which point the benefit of treatment suddenly appears or disappears. By definition, there must be a gradient of treatment response across the full spectrum of ischaemia. This gradient will apply to both subjective “soft” (e.g., symptoms) and objective “hard” (e.g., death) endpoints.
Initial FFR validation studies tested the index against non-invasive tests including exercise electrocardiogram testing, myocardial perfusion scintigraphy and dobutamine stress echocardiography. They showed an optimal threshold of 0.75, whereby, if FFR was positive, one or more of the other functional tests were also positive24. This was termed the “ischaemic threshold”. The higher “clinical threshold” of FFR ≤0.80 was introduced in the clinical outcome trials to provide a “safety margin”. It was felt that raising the threshold would increase the negative predictive value of this biological measurement compared to the previous cut-point of FFR <0.757.
Inadvertently, previous observations of decreased specificity in the FFR range of 0.75 to 0.80 and shifting thresholds have now led to the concept of a physiological “grey zone”. Within the “grey zone”, consideration of other diagnostic modalities and risk versus benefit analysis should be used to add clarity before a decision is made to pursue intervention, especially since measurements clustering around 0.77 to 0.83 demonstrate particular biological variability with respect to decision-making cut-points25.
To define the cut-points for physiological measurements at rest (iFR), validation against FFR was required; therefore, the perpetuation of dichotomy continued. In the early development of the technique, iFR thresholds of 0.83 and 0.86 were reported to provide good agreement with an FFR of 0.8026,27. Before the clinical outcome data were available, hybrid algorithms were used for iFR28. For iFR <0.86, revascularisation was advised; for iFR >0.93, deferral was recommended and, for iFR 0.86 to 0.93, hyperaemic FFR measurements were required for adjudication, resulting in avoidance of hyperaemia in 57% of patients and 95% agreement with an FFR-only strategy28. Higher iFR thresholds of ≤0.89 (with diagnostic accuracy of 82.5%)29 were eventually adopted for use in the landmark clinical trials16,17.
Although decision making (to treat versus not to treat) is regarded as binary, the process of dichotomisation of biological measurements removes most of the information content and assumes discontinuity in response once these interval boundaries are crossed23. Hence, despite clinical trials adopting these thresholds, in clinical practice ischaemia should be regarded as a continuum in the context of a patient rather than as a definitive cut-off.
CONSENSUS GUIDELINES
The importance of assessing the functional relevance of a coronary artery stenosis is central to European Society of Cardiology (ESC) guidance on decision making for myocardial revascularisation in stable CAD. Invasive functional testing, with FFR or iFR, has a class I, level of evidence “A” endorsement from the ESC to assess the severity of an intermediate lesion where evidence of ischaemia from non-invasive tests is not available1. Furthermore, FFR guidance of PCI is recommended with class IIa, level of evidence B for patients with multivessel disease undergoing PCI. Importantly, the ESC acknowledges a gap in the evidence base which prevents clear recommendations for FFR for the assessment of non-infarct-related artery (non-IRA) disease in the setting of ST-elevated MI (STEMI) with bystander disease30. Much like clinical trial design, nuanced directives are difficult for consensus guideline documents; therefore, dichotomous cut-offs are used but clinical judgement remains paramount. It is incumbent upon our guideline committees to continue to refine and reassess these recommendations in the light of increasing high-quality bias-resistant randomised controlled trial data.
Physiology to guide PCI
CHANGING DIRECTION FOR CORONARY PHYSIOLOGY
As the limitations of a dichotomised approach to the interpretation of ischaemia tests become increasingly apparent, the application of coronary physiology is moving away from simple justification of PCI using binary cut-points towards real-time guidance of PCI strategy. Advances in pressure wire software have allowed pressure wire pullback and co-registration technology to provide operators with in-cath lab physiological mapping to inform stenting strategy for the best haemodynamic result. This allows the identification of lesions with the largest pressure gradients and therefore the most favourable PCI targets. It also allows the operator to differentiate between focal stenoses, amenable to PCI, and diffuse disease which may result in an abnormal distal vessel physiology assessment but may not represent a technically suitable PCI target.
LIMITATIONS OF FFR PULLBACK
Pressure wire pullback using FFR is limited by the phenomenon of haemodynamic interdependence (“cross-talk”) between serial stenoses or diffuse disease in the same vessel. This occurs because, under conditions of stable hyperaemia, the maximal flow achieved across one stenosis is limited by the haemodynamic characteristics of a sequential stenosis, resulting in a tendency to underestimate true stenosis severity for each of the lesions31,32. The problem this poses is that, once one stenosis is treated, hyperaemic flow, and therefore the pressure drop, across the second stenosis will increase. These haemodynamic characteristics make FFR less favourable for pullback assessments, particularly in the presence of tandem stenoses or diffusely diseased vessels.
iFR PULLBACK WITH CO-REGISTRATION
Under resting conditions, coronary flow is preserved across a wide spectrum of stenosis severity until a vessel is subtotally occluded33. Consequently, the benefit of performing pressure wire pullback assessments under resting conditions, without prerequisite hyperaemia, is that the assessment is less vulnerable to haemodynamic interdependence between sequential stenoses within the same vessel.
In this way, resting indices such as iFR, which are calculated on a beat-to-beat basis, permit a physiological map to be created with high spatial resolution during pressure wire pullback34. Through accurate quantification of the pressure gradient at each point within a vessel, iFR pullback can guide intervention by highlighting the stenoses which contribute the greatest pressure drop within the vessel. Equally important is the use of this technology to prevent unnecessary stenting of vessel segments which may angiographically appear significant, but do not contribute meaningfully to ischaemia in the vascular territory. iFR pullback, therefore, allows the pattern of CAD to be characterised as focal, diffuse or a mixed distribution.
The next derivation of this technology, iFR co-registration, is now able to track the movement of the pressure wire through the vessel in real time under continuous fluoroscopy. The data from an iFR pullback manoeuvre can be co-registered to the coronary angiogram, creating a physiological roadmap, whereby the pressure drop at any given stenosis is depicted visually on the patient’s angiogram (Figure 1A),32. Furthermore, iFR co-registration enables modelling of custom stenting strategies in order to predict the post-PCI physiological result to a high degree of accuracy. This allows operators to plan their preferred stenting strategy in order to optimise the physiological gain per unit of stented segment (Figure 1B).
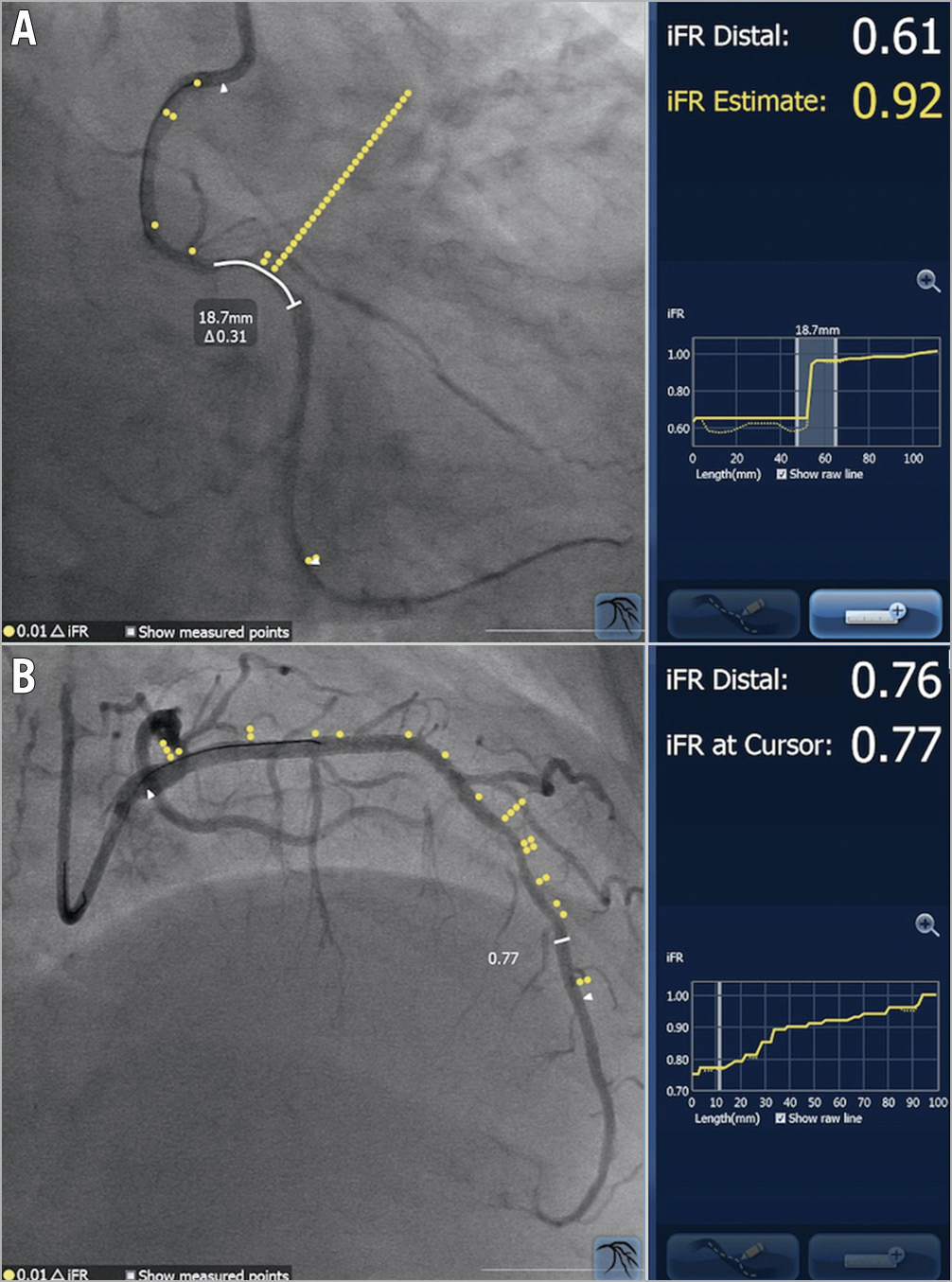
Figure 1. iFR co-registration images. The yellow dots overlying each angiogram correspond to the location of a 0.01 iFR pressure drop. A) A focal stenosis in the right coronary artery. The operator has specified the planned stented segment, which results in a predicted physiological gain of 0.31 iFR units, resulting in an estimated post-PCI iFR of 0.92 for the vessel. B) A diffusely diseased left anterior descending artery. The cursor may be moved to any given location in the vessel to determine the estimated iFR at that position.
Post-PCI physiology
FAILURES OF STANDARD APPROACH
Cardiologists have accepted that revascularisation decisions made on the visual appearance of the coronary angiogram alone are flawed. However, the success of coronary intervention is widely adjudicated with nothing more than this limited assessment technique. More rigorous measures of procedural success have not become well established and, as such, a contradiction exists between the methods we deem appropriate for justification of PCI and the methods by which we judge the success of our work. Despite angiographic success, further interrogation of stented lesions with optical coherence tomography (OCT), intravascular ultrasound (IVUS) and coronary physiology has shown that patients may have suboptimal PCI results, with residual ischaemia occurring in 18-25% of patients35,36. Frequency of recurrent angina with PCI guided by the angiogram alone can be as high as 50% at short-term follow-up3 and around 30% at one year37. With physiology-guided revascularisation, one in five patients still reports symptoms of angina, and major adverse cardiac events (defined as death, non-fatal MI, and repeat revascularisation) remain relatively frequent after revascularisation with PCI (13% at 1 year and 28% at 5 years)12. While the link between invasive physiology and symptoms requires further study, if we utilise physiology to justify revascularisation there may be a benefit to using the same tools to judge the success of coronary intervention.
RATIONALE FOR POST-PCI PHYSIOLOGY ASSESSMENTS
Observational evidence exists linking post-PCI physiology with clinical outcomes. A number of unblinded studies have now suggested that post-PCI physiology is independently associated with major adverse cardiovascular events, in particular target vessel failure (Table 5).
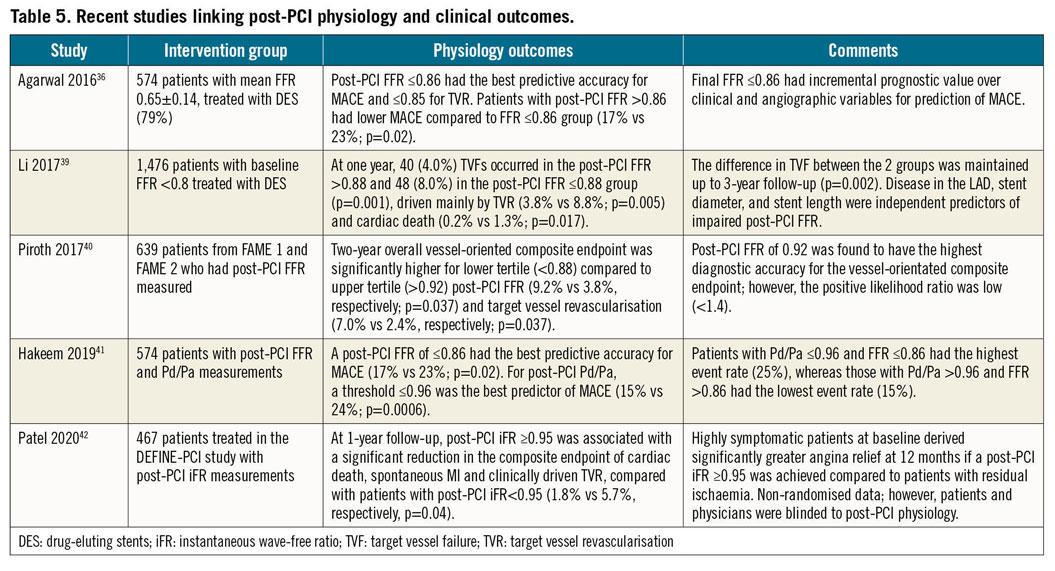
Although various cut-off values have been suggested for post-PCI physiology, a meta-analysis of 970 lesions from 10 studies found that there was an independent and continuous relationship between post-PCI FFR and major adverse cardiovascular events without clear discontinuity in outcomes (Cox hazard ratio: 0.86, 95% confidence interval [CI]: 0.80 to 0.93; p<0.001)38. Interestingly, only post-PCI FFR retained prognostic value (adjusted Cox hazard ratio: 0.90, 95% CI: 0.82 to 0.99; p=0.032) compared to pre-PCI FFR (adjusted Cox hazard ratio: 0.97, 95% CI: 0.92 to 1.02; p=0.28) after adjustment for pre- and post-PCI measurements. Hence, in corroboration with the concept of continuous ischaemic risk, further intervention in an effort to reduce ischaemia when post-PCI FFR values are suboptimal should be attempted. Individual studies are shown in Table 5,36,39,40,41.
It is pertinent to consider, however, that the post-PCI cut-off values in the individual studies are derived from observational data and post hoc analysis. It is therefore likely that there is a continuous post-PCI ischaemia spectrum with no dichotomous cut-point for an optimum post-PCI result. However, in order to test the use of post-PCI physiology formally, pre-specified optimum thresholds will be needed for clinical trials, just as was necessary with pre-PCI physiology trials. In the available post-PCI physiology studies, thresholds have been variously defined with margins above the currently accepted ischaemic threshold (FFR ≤0.80, iFR ≤0.89). A key limitation of the available evidence base is that blinded evaluation of post-PCI physiology among operators and patients was not always mandated. Hence, certain endpoints, such as urgent revascularisation, may be influenced by knowledge of post-PCI physiological results because they are vulnerable to bias.
Most studies have not evaluated further intervention to optimise physiological measurements, as these procedures were deemed angiographically successful. Therefore, the mechanisms for suboptimal post-PCI physiology remain unknown. Before incorporating the logical construct of post-PCI physiology into guideline recommendations, randomised controlled trials are needed to define the utility of post-PCI physiology and the additive benefit of subsequent intervention on clinical endpoints.
MECHANISMS FOR POST-PCI ISCHAEMIA
Determining the mechanism of post-PCI ischaemia is fundamental to an operator’s ability to target further intervention and optimise the result. Equally important is the possibility that discovering the mechanism of post-PCI ischaemia may confirm that additional treatment would be futile in achieving further incremental gains in the haemodynamic properties of the vessel, such as in the setting of diffuse disease. This may save the patient unnecessary additional intervention.
An excellent final angiographic result may frequently co-exist with residual post-PCI ischaemia. Clues regarding the mechanism of a poor physiological result may not be forthcoming from the angiogram alone. As a lumenogram, the coronary angiogram often hides diffuse disease within an artery: the “normal” reference diameter of the vessel may therefore be underestimated and, without intravascular imaging, this will not be appreciated. Secondly, following PCI, an increase in flow to the distal vessel may result in an angiographically moderate tandem stenosis taking on greater physiological significance.
Imperfections with PCI itself must also be recognised as important causes of post-intervention ischaemia. At worst, a geographic miss of the culprit lesion may have occurred (Figure 2). Experienced operators remain vulnerable to this if ancillary tools such as pullback and co-registration are not used to guide PCI by localising the regions of maximal pressure loss. More commonly, undersized or underexpanded stents may impair the physiological result and may be confirmed with intravascular imaging. Finally, proximal or distal stent edge dissections may meaningfully impede flow to the distal vessel and should always be considered in the setting of post-PCI ischaemia.
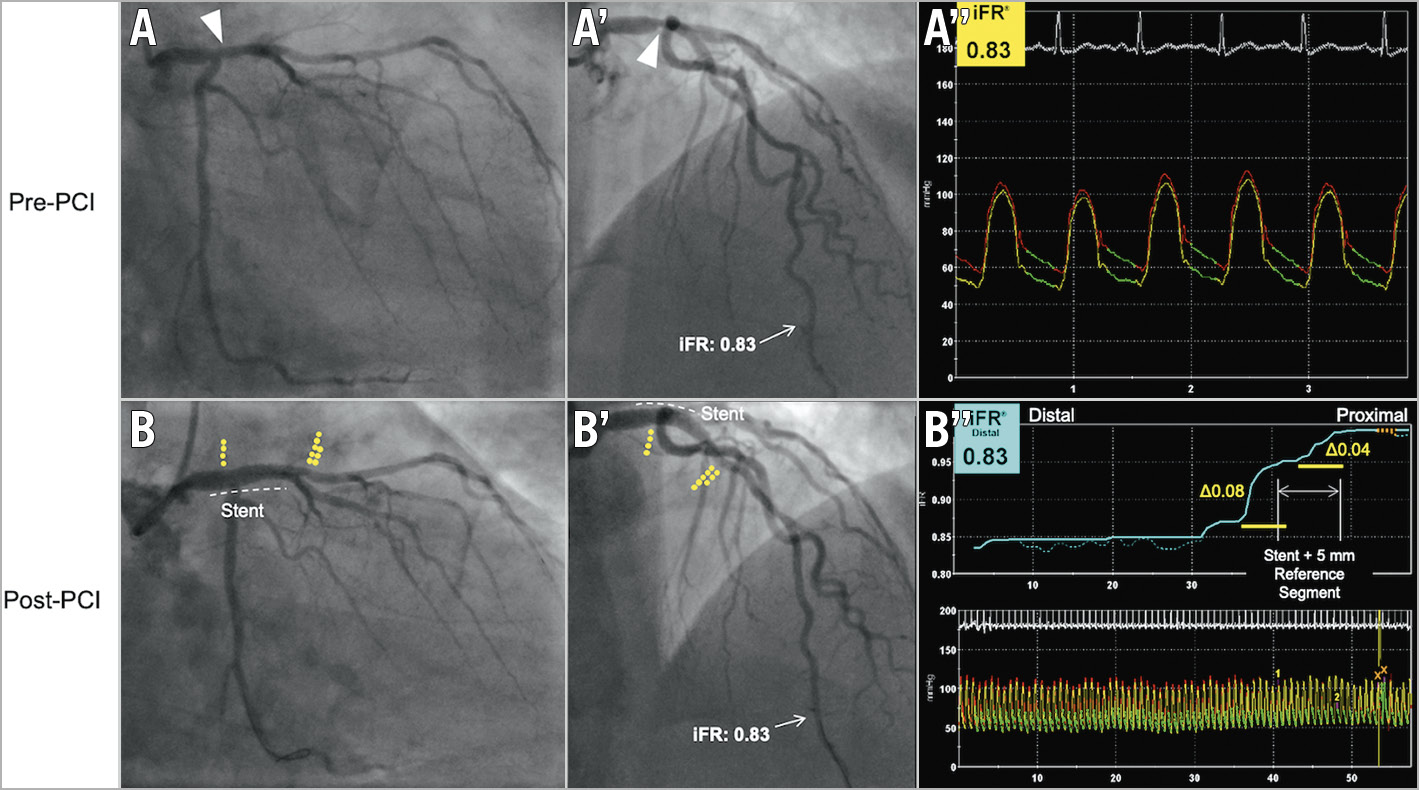
Figure 2. An example of a geographic miss during PCI to the proximal left anterior descending (LAD) artery and left main stem (LMS). A) A moderate stenosis noted in the distal left main stem. A’) The distal iFR in the LAD is 0.83. The operator performs PCI to the LMS, extending into the proximal LAD. B) Post-PCI iFR co-registration shows localised focal pressure loss at the entry of the stent, but also a second, larger pressure loss distal to the stented segment. B’) The distal iFR remains the same (0.83) as pre-PCI due to geographic miss.
The DEFINE PCI (Physiologic Assessment of Coronary Stenosis Following PCI) study examined the frequency and mechanism of residual post-PCI ischaemia using blinded post-PCI iFR-pullback assessments35. After angiographically successful PCI, 24.0% of patients in the study were found to have residual ischaemia, as defined by post-PCI iFR <0.90. When this was stratified by the pattern of disease on iFR pullback, 81.6% had one or more focal areas of pressure loss and only 18.4% of patients had diffuse disease. Where residual focal stenoses existed, these were geographically distributed within (38.4%), proximal (31.5%) and distal (30.1%) to the stented segment. These residual focal pressure gradients were poorly correlated to core lab quantitative coronary angiography (QCA) analysis of minimum lumen diameter or percent residual stenosis, demonstrating the inability of coronary angiography to determine physiological lesion severity. DEFINE PCI therefore served to prove that post-PCI residual ischaemia is common, even after contemporary, angiographically successful PCI. Secondly, it found that residual focal lesions, potentially amenable to further treatment, were most commonly the cause.
The one-year outcomes of DEFINE-PCI showed that achieving a post-PCI iFR ≥0.95 was associated with a significant reduction in a composite endpoint of cardiac death, spontaneous MI, or clinically driven target vessel revascularisation42, albeit with the limitations of an observational study design.
TECHNICAL CONSIDERATIONS
PITFALLS IN PHYSIOLOGICAL ASSESSMENTS
Adherence to meticulous technique is the cornerstone of accurate physiological assessments, in the pre- or post-PCI setting. Pitfalls in the optimal assessment technique are frequently encountered, but are often easily resolved. In Table 6 and Figure 3, we highlight the most common errors for any physiological assessment, their physiological consequences, and techniques to resolve the problem.
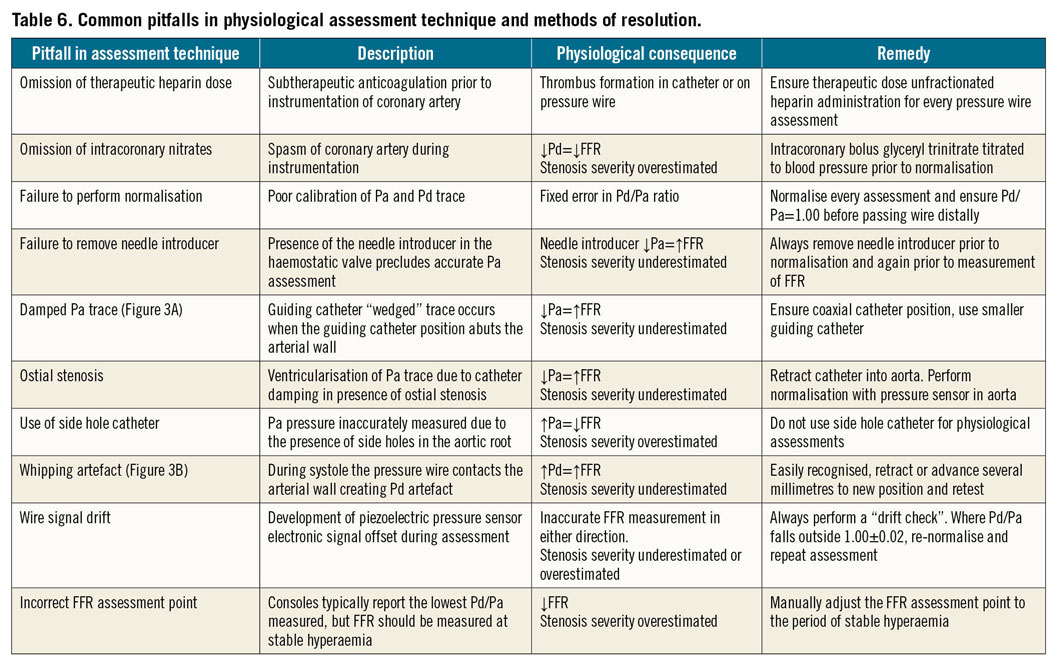
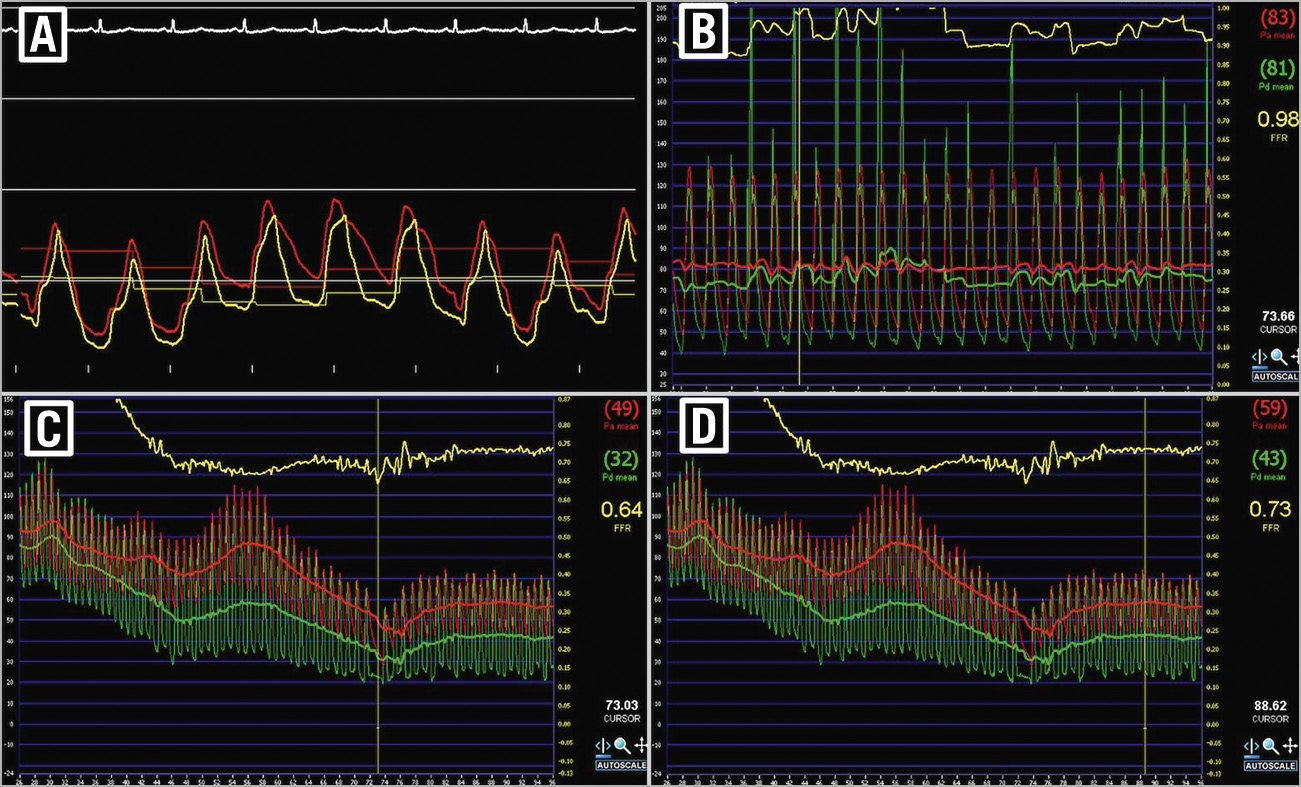
Figure 3. Pitfalls in the assessment of coronary physiology. A) Example of damped Pa trace with a characteristic waveform. B) Example of whipping artefact. C) Example of an incorrect cursor position to measure FFR. The console may default measurement to the lowest Pd/Pa value. However, this position does not correspond to stable hyperaemia and will overestimate stenosis severity. D) The cursor has now been adjusted to ensure that FFR is measured at a period of stable hyperaemia.
TECHNICAL CONSIDERATIONS SPECIFIC TO POST-PCI PHYSIOLOGICAL ASSESSMENTS
Coronary vasospasm is common following PCI as the vessel reacts to stenting. It follows that administration of intracoronary nitrates before repeating physiological assessment is particularly pertinent after PCI, regardless of the physiological assessment technique.
In the post-PCI setting, advancement of the pressure wire may be technically challenging, particularly through more complex stented segments. Passing the pressure wire alongside the workhorse wire can be an effective strategy. Consideration should also be given to the possible risk of advancing a pressure wire through concealed ruptures or dissections which may not be apparent angiographically if there is significant post-PCI haziness. For this reason, a potential advantage may be offered by the use of a miniaturised dual lumen catheter for wire exchange distal to the stented segment, although damping of the proximal aortic pressure tracing through the guiding catheter affects the accuracy of this technique. Dedicated microcatheter-based pressure sensors have been developed, but systematically overestimate lesion severity due to a pressure effect of the catheter across the vessel segment under assessment43. To avoid these sources of inaccuracy, a small outer diameter over-the-wire microcatheter may be used to exchange the workhorse wire for a pressure wire, with use of a trapping balloon technique inside the guiding catheter to remove the microcatheter. This more complex solution is helpful to ensure accurate haemodynamic measurements.
Previous discussion has centred upon the validity of NHPRs in the post-PCI setting. A theoretical concern was raised regarding the period of hyperaemia that follows balloon inflation during PCI. NHPRs are susceptible to residual hyperaemia, which will reduce the NHPR result if resting conditions are not restored before measurement. However, based on current evidence, hyperaemia following transitory coronary occlusion only persists for approximately one minute on average44. Hence, in the time it takes to carry out physiological assessments post PCI, resting conditions are typically restored.
On a more practical note, the purpose of carrying out post-PCI physiological assessment is twofold – to diagnose a suboptimal haemodynamic result and, secondly, to elicit its cause. We propose that for the first objective available data would suggest that either FFR or an NHPR could be used. However, where post-PCI ischaemia is found, an NHPR is more robust for performance of pullback and co-registration which will provide focus on the underlying mechanism of ischaemia. The use of a hyperaemic pressure ratio for pullback assessments in the post-PCI setting is likely to be vulnerable to the same limitations as in the pre-PCI setting, namely haemodynamic cross-communication between diseased segments of the same vessel32.
METHODS OF OPTIMISATION
For post-PCI physiology to be useful, operators must be equipped with a strategy to intervene on the results of a suboptimal post-PCI physiology result. This strategy will vary on a case-by-case basis but, when performed effectively, post-intervention optimisation can reclassify vessels from “ischaemic” to “non-ischaemic” physiological criteria. In the largest study to date, Agarwal et al36 examined post-PCI FFR in 574 patients (664 lesions); 21% of these lesions had a post-PCI FFR ≤0.81, again highlighting the frequency of post-PCI ischaemia. Repeat interventions were carried out in the majority of this group, including post-dilation of the implanted stent (42%), another stent implantation (33%) and further stenting with further balloon post-dilation (18%). As a result of these interventions, the mean post-PCI FFR in this group increased from 0.78±0.07 to 0.87±0.05.
As previously discussed, the optimal method for any further post-PCI intervention will depend upon the mechanism of the suboptimal result. The importance of intravascular imaging with IVUS or OCT to determine this mechanism cannot be overstated. Reliance on angiographic views alone to assess the success of revascularisation is particularly limited in the setting of post-PCI haziness which may preclude visualisation of concealed ruptures or dissections. Operators must therefore be confident with the interpretation of post-PCI intravascular imaging in order to determine an effective optimisation strategy. Stent underexpansion, malapposition and entry and exit site dissections may only be identified with this approach. Randomised data are now available to support a reduction in target vessel failure when PCI is guided by intravascular imaging as opposed to angiography alone45,46. Trials specific to post-PCI imaging optimisation have not been conducted; however, it is a reasonable assumption that the benefits would extend to this setting. Ultimately, synergistic data from intravascular imaging and adjunctive tools such as physiology co-registration will afford the operator the best opportunity to target any post-PCI intervention to where it is most needed (Central illustration).
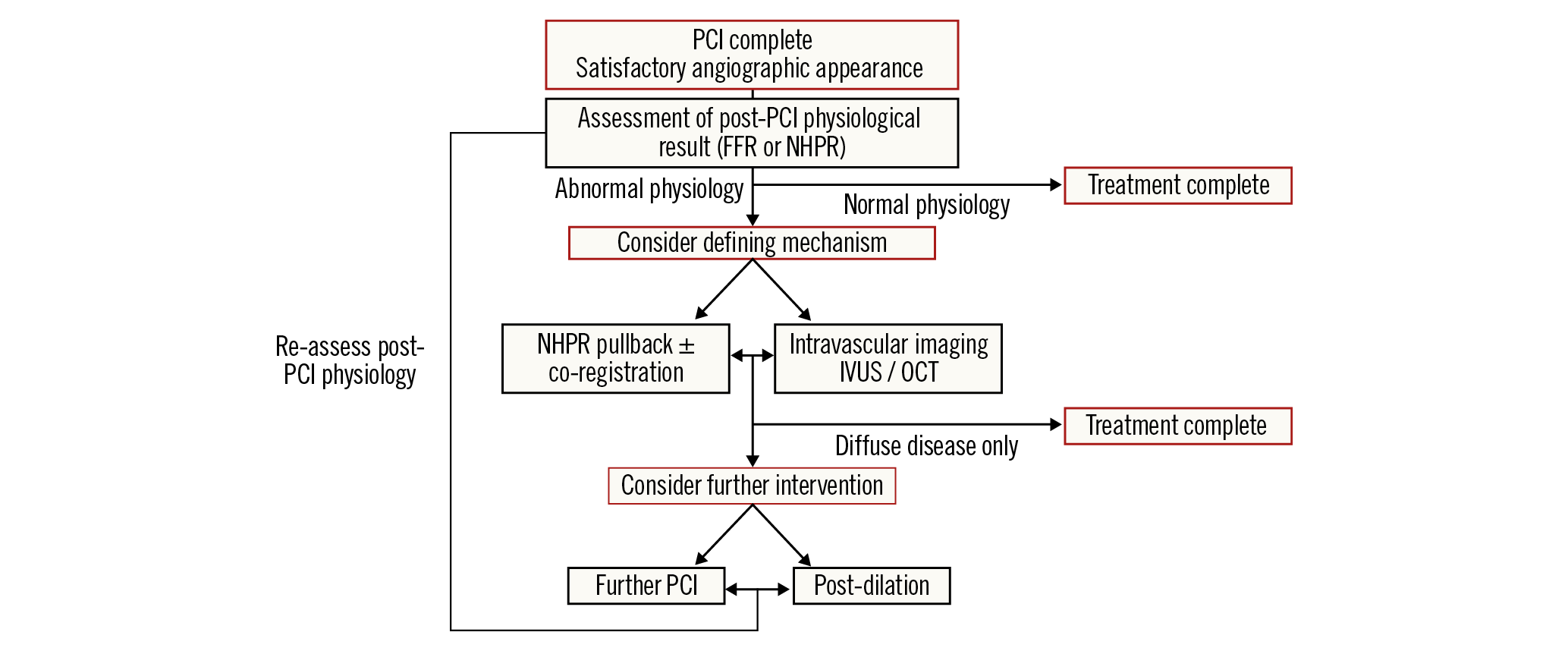
Central illustration. Proposed potential algorithm for post-PCI physiological assessment and treatment.
Unresolved questions regarding myocardial ischaemia
Recent randomised controlled trials have cast doubt on the practice of ischaemia testing as a method of selecting patients able to benefit from revascularisation. It is important to consider these broader data to contextualise the evolving roles of invasive coronary physiology.
Scientifically it seems logical that the degree of ischaemia may be associated with clinical outcomes. Early observational studies showed that, in order to obtain a survival benefit from revascularisation over and above medical therapy, greater than 10% of the myocardium needed to be ischaemic47. However, this was based on retrospective data assessing the difference in outcomes according to the degree of ischaemia with patients allocated to two groups depending on whether or not PCI was performed. Patients were not randomised, and the data were therefore potentially confounded. It is quite possible that the drivers for conservative management were unmeasured and highly influential. Nevertheless, these limited data informed clinical guidelines and set the precedent for widespread practice of ischaemia-guided revascularisation1,48.
Subsequent randomised data emerged from a nuclear substudy of the COURAGE (Clinical Outcomes Utilising Revascularisation and Aggressive Drug Evaluation) trial49,50. While a substudy of patients who underwent serial myocardial perfusion scintigraphy demonstrated that adding PCI to OMT resulted in greater reduction in ischaemia compared with OMT alone50, there was no evidence that PCI reduced the primary endpoint of death or MI in patients with moderate to severe ischaemia49.
The ISCHEMIA (International Study of Comparative Health Effectiveness with Medical and Invasive Approaches) trial was designed to answer definitively the question of whether an early invasive strategy offered additive clinical benefit versus conservative management in patients with moderate to severe ischaemia for hard clinical endpoints2. Overall, there was no significant difference between an initial invasive approach with cardiac catheterisation and revascularisation in comparison to a conservative approach for a five-component composite endpoint of death, MI, hospitalisation for heart failure or unstable angina and resuscitated cardiac arrest (HR 0.93, p=0.34). In the group randomised to an early invasive approach, there was a significant reduction in spontaneous MI when compared to the control arm, a benefit that was offset by a significant increase in the risk of periprocedural MI which resulted in no difference between the groups in overall MI or cardiovascular death. This highlights the potential trade-off in risk between early (periprocedural) and late (spontaneous) MI that may be observed with an invasive approach. A similar reduction in spontaneous MI was also observed in the extended follow-up of the FAME 2 trial12. While the implications of a spontaneous MI may be of greater clinical relevance than a periprocedural MI, these differences have not translated into a difference in the overall rates of cardiovascular death in those trials. Longer-term follow-up will be required to understand the clinical implications of these results fully.
These data should allow us to be confident that treatment of myocardial ischaemia with OMT is safe and effective. PCI has been prognostically neutral in the patient populations studied. The principal driver for revascularisation in stable coronary disease is, therefore, not the presence of residual ischaemia but the persistence of angina, despite OMT.
A simple linear relationship between the severity of ischaemia and symptomatic angina again seems scientifically logical. However, clinical practice would suggest that this reasonable assumption is misplaced. The physiology-stratified sub-analysis of the ORBITA (Objective Randomised Blinded Investigation with optimal medical Therapy of Angioplasty in stable angina) trial showed that the greater the degree of ischaemia measured by FFR and the iFR at baseline, the greater the extent of myocardial perfusion improvement seen on stress echocardiography following revascularisation51. However, while these tests of ischaemia were very well correlated, the correlation of baseline ischaemia with exercise time and the symptom and quality-of-life endpoints of ORBITA were less clear51. Further research in broader patient populations is required to understand more fully the relationship between ischaemia and symptom severity. The clearest message of ORBITA was the importance of blinding, with placebo control, when symptoms are the endpoint under investigation. Unblinded assessments of symptoms in trials of revascularisation should be disregarded, as knowledge of treatment allocation profoundly affects physician and patient reporting10.
Taken together, it is incumbent upon all in our community to reflect upon these trial results and to consider carefully the indication for PCI on an individual patient basis. It also seems timely now to reconsider the value of ischaemia testing. If the presence of ischaemia does not predict poor clinical outcomes and these outcomes are not mitigated by resolution of ischaemia, what is the value of these tests? Their role may be predominantly in the diagnosis of symptoms that are the result of cardiac disease and the guidance of where to treat rather than in selecting those patients who stand to benefit the most. Most importantly, in the absence of symptoms, they may have a limited role in the prediction of risk.
Future directions and recommendations
A number of upcoming trials will investigate the association between post-PCI physiology and clinical outcomes. DEFINE-GPS (Distal Evaluation of Functional performance with Intravascular sensors to assess the Narrowing Effect: Guided Physiologic Stenting [NCT04451044]) is a randomised controlled trial that aims to randomise ~3,000 participants to PCI guided by iFR co-registration or angiography alone52. The primary endpoint will be major adverse cardiac events or rehospitalisation for unstable angina at two years. The DEFINE-GPS trial will improve our understanding of the utility of NHPR co-registration techniques in guiding PCI and subsequent intervention as coronary stenting is increasingly applied to more complex patients.
The double-blind ORBITA-2 trial (NCT03742050) is currently randomising patients with single and multivessel CAD to PCI versus placebo for the treatment of stable angina. Post-PCI physiological assessments will be performed allowing, for the first time, the association between post-PCI physiological results and subsequent angina relief to be tested in a cohort of patients blinded to treatment allocation.
ORBITA-STAR (NCT04280575) is an “n-of-1” study assessing whether placebo-controlled verification of angina symptoms during induced ischaemia can more accurately predict a positive response to PCI. This may offer the potential to maximise the therapeutic efficacy of PCI by identifying the patients most able to benefit symptomatically from treatment.
The role of artificial intelligence is increasing in prominence in a number of settings in cardiology; coronary physiology is no exception to this trend. It has been shown that around 30% of coronary physiology data is excluded after core laboratory assessment due to signal drift or abnormal waveforms53. Artificial intelligence has been identified as an approach to real-time identification of abnormal waveforms to alert the interventionist and avoid diagnostic errors in measurements. Arterial waveform analysis using neural networks has been shown to identify rapidly and accurately (98.7% to 99.4% accurate) the presence of damping, demonstrating how machine learning can aid in ensuring the quality and safety of routine clinical practice54. Future research will determine whether neural networks can be trained to guide the optimum site of revascularisation based on physiology and pullback data.
We also envisage that novel techniques for physiological assessment that eliminate additional pharmacology or even coronary wire use may supersede current practice and further enhance both operator and patient experiences. Quantitative flow reserve (QFR), initially described in the FAVOR (Functional Diagnostic Accuracy of Quantitative Flow Ratio in Online Assessment of Coronary Stenosis) pilot study, uses a three-dimensional angiographic reconstruction of the stenosed vessel and the contrast flow frame count to make an assessment of FFR. Studies have shown promising results regarding the ability of QFR to identify the presence of functionally significant stenosis55, and a possible prognostic value of post-PCI QFR56,57. This, along with FFR-CT58, may offer newer technologies that encompass anatomic and physiological assessment of CAD with reduced procedural risk.
When considering prognostic endpoints, it must be remembered that many adverse cardiac events originate from angiographically and physiologically non-significant lesions. The PROSPECT (Providing Regional Observations to Study Predictors of Events in the Coronary Tree) study showed that, after acute coronary syndrome (ACS), the majority of non-culprit lesions responsible for major adverse cardiac events during follow-up were angiographically mild, non-flow-limiting lesions at baseline, but with high-risk features such as thin-cap fibroatheromas or large plaque volumes4. More recently, the LRP (lipid-rich plaque) and PROSPECT ABSORB (Providing Regional Observations to Study Predictors of Events in the Coronary Tree II combined with a randomised, controlled, intervention trial) studies have demonstrated the utility of intravascular imaging including near-infrared spectroscopy (NIRS) for the identification of vulnerable lipid-rich plaques, which may be physiologically non-flow-limiting, but pose high risk of rupture and associated MACE59,60. Whether preventative treatment of these lipid-rich plaques can translate into improved clinical outcomes remains to be determined and requires further dedicated trials. Nevertheless, together these data suggest that physiological optimisation of PCI can only achieve so much: development of clinical tools to identify the vulnerable plaque will be pivotal for meaningful steps to be made towards the aim of cardiac risk reduction through revascularisation. These tools, in combination with truly optimum medical therapy, will allow prognostic endpoints to be optimised in stable CAD.
Conclusion
More than 30 years ago, the concept of FFR was developed in order to help coronary interventionalists decide whom to treat with revascularisation. It was an attractive technique which offered immediate numerical results and unambiguous interpretation with a clear-cut threshold for treatment.
More recently, based on randomised data including many thousands of patients, our understanding of myocardial ischaemia and coronary physiology and their association with prognostic and symptomatic endpoints in stable coronary artery disease has begun to evolve. The simplistic relationships between myocardial ischaemia, coronary revascularisation and long-term outcomes are much more complex than previously thought.
Coronary physiology is now evolving to support new roles in the catheterisation laboratory, and its use to optimise physiological results of PCI should be encouraged. Nevertheless, randomised, blinded data examining these new roles should be considered essential to their long-term success as tools to optimise clinical outcomes for patients.
Acknowledgements
C. Rajkumar is supported by a Clinical Research Training Fellowship from the Medical Research Council (MR/S021108/1). The authors acknowledge the support of the Imperial College London British Heart Foundation Centre of Research Excellence.
Conflict of interest statement
R. Al-Lamee has received speaker’s honoraria from Philips Volcano and Menarini Pharmaceuticals. A. Jeremias has received institutional funding (unrestricted education grant) from, and serves as a consultant for, Philips Volcano and Abbott Vascular, and is a consultant for Boston Scientific and ACIST Medical Systems. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

