
Abstract
Aims: Myocardial injury reflected by a post-procedural increase of serum troponin I (TnI) occurs frequently during transcatheter aortic valve implantation (TAVI). It is potentially caused by intraprocedural hypotension, periprocedural coronary microembolisation and post-procedural (para)valvular leakages (PVLs). We invasively assessed coronary flow dynamics including coronary flow velocity reserve (CFVR), embolic high-intensity transient signals (HITS) as well as rapid pacing induced hypotension and post-procedural PVLs to determine their contribution to post-procedural TnI increases.
Methods and results: In 15 transfemoral TAVI patients, TnI was measured serially, and cardiac MRIs with late gadolinium enhancement (LGE) were performed pre- and post-interventionally. There were no significant correlations between coronary flow dynamics, CFVR and the area under the curve (AUC) of TnI over 72 hours. Despite the detection of HITS in all patients and during all procedural steps, there was also no correlation between the amount of HITS and the AUC of TnI. However, there were positive correlations between the duration of rapid pacing as well as the time of subsequent blood pressure recovery and the AUC of TnI. Both LGE and more than mild PVL were observed in a single case only.
Conclusions: Myocardial injury after TAVI appears to be related more to hypoperfusion-induced ischaemia than to periprocedural microembolisation.
Abbreviations
AUC: area under the curve
AVA: aortic valve area
bAPV: baseline average peak velocity
BAV: balloon aortic valvuloplasty
CFVR: coronary flow velocity reserve
EuroSCORE: European System for Cardiac Operative Risk Evaluation
hAPV: hyperaemic average peak velocity
HITS: high-intensity transient signals
ICD: intracoronary Doppler
LAD: left anterior descending
LGE: late gadolinium enhancement
MRI: magnetic resonance imaging
NYHA: New York Heart Association
PVL: paravalvular leakage
SBP: systolic blood pressure
STS: Society of Thoracic Surgeons
TAVI: transcatheter aortic valve implantation
THV: transcatheter heart valve
TnI: serum troponin I
Introduction
Transcatheter aortic valve implantation (TAVI) is regularly associated with some degree of myocardial injury, as reflected by small yet significant post-procedural increases of serum troponin concentrations1-4. Importantly, the extent of myocardial injury appears to be an independent predictor for mortality after TAVI2,5. However, the aetiology of such myocardial injury has not been precisely identified. Episodes of profound hypotension with consequent myocardial ischaemia during valvuloplasty and stent-valve implantation or mechanical trauma to the basal myocardial septum by the implanted bioprosthesis have been considered as possible causes2. Procedural embolisation of plaque and valvular debris into the coronary (micro)circulation could be another origin of myocardial injury, reminiscent of recently reported cerebral embolisation4,6,7. In addition, in patients with a relevant (para)valvular leakage (PVL), decreased myocardial perfusion pressure may contribute to increases of serum troponin I (TnI)7,8.
The aim of our hypothesis-generating study was to evaluate the contribution of coronary flow dynamics including coronary flow velocity reserve (CVFR), embolic high-intensity transient signals (HITS), rapid pacing induced hypotension and post-procedural PVLs to myocardial injury during transfemoral TAVI using intracoronary Doppler (ICD) monitoring of the blood flow in the left anterior descending (LAD) coronary artery, serial TnI measurements and pre- and post-interventional cardiac magnetic resonance imaging (MRI).
Methods
STUDY POPULATION
Between September 2012 and August 2013, 15 patients with severe, symptomatic aortic valve stenosis, who underwent transfemoral, balloon-expandable TAVI using the SAPIEN XT (Edwards Lifesciences Inc., Irvine, CA, USA), were included. The decision for TAVI was made by the institutional Heart Team, and the study was approved by the local ethics committee (reference number: 12-5090-BO). All patients gave written informed consent, and the study conformed to the principles of the Declaration of Helsinki.
Patients with prior coronary artery bypass grafting, LAD stenosis, recent myocardial infarction and preoperative TnI concentration above the upper normal reference limit of 0.1 ng/ml were excluded from the study.
TAVI PROCEDURE
TAVI procedures were performed in a hybrid operating room under anaesthesiologist-controlled conscious sedation with invasive blood and pulmonary artery pressure monitoring to assure haemodynamic stability9. Particular care was taken to maintain a systolic blood pressure >90 mmHg prior to the initiation of rapid pacing in order to prevent subsequent hypotension by administration of colloidal or crystalloid solutions and low-dose vasoactive support, if required.
INTRACORONARY DOPPLER
A 0.014-inch Doppler wire connected to a Doppler console (FloWire® and FloMap® System; Volcano Therapeutics, Inc., Rancho Cordova, CA, USA) was placed in the mid LAD via radial or brachial access for serial CFVR measurements and for continuous blood flow velocity monitoring with detection of HITS.
CORONARY FLOW DYNAMICS
Adenosine-recruitable CFVR was measured serially at baseline, after valvuloplasty and after TAVI using intracoronary bolus administration of 36 μg adenosine for the induction of hyperaemia. CFVR was calculated as the ratio of maximal hyperaemic average peak velocity (hAPV) and baseline average peak velocity (bAPV).
DETECTION OF HITS
The principles of detection of microemboli with a multifrequency Doppler wire have been described previously10, and microembolic HITS were defined by established criteria11,12. By convention, a 1 s episode of microembolic shower signals was counted as 10 microemboli13, and all HITS recorded during contrast injections were excluded from the analysis since they may have reflected microbubbles14.
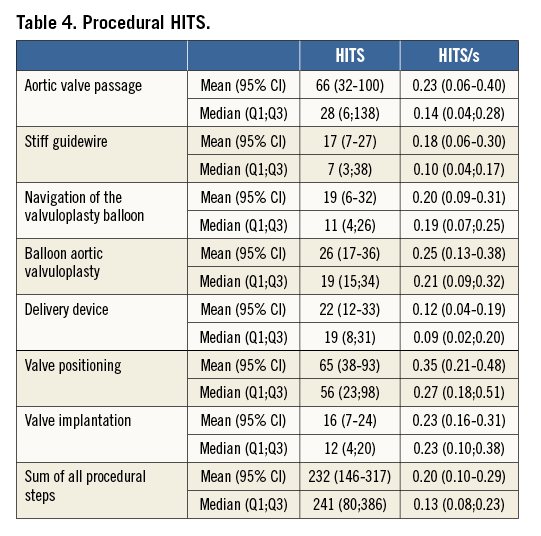
The counting of HITS (Figure 1) was performed offline from VHS tape recordings dividing the procedure into the following phases: 1) retrograde wire passage of the aortic valve, 2) introduction of the stiff guidewire into the left ventricular apex, 3) navigation and placement of the balloon for valvuloplasty, 4) balloon valvuloplasty, 5) introduction and propagation of the loaded delivery device towards the aortic annulus, 6) positioning, and 7) implantation of the stent valve. In addition, HITS/s were calculated for each step.
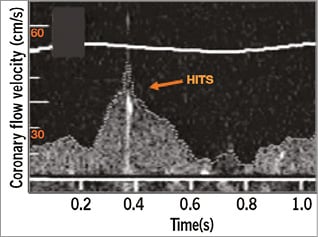
Figure 1. High-intensity transient signals (HITS). Example of a high-intensity transient signal occurring during diastole (arrow).
Interobserver agreement was assessed for three observers who independently analysed the Doppler recordings for HITS, unaware of TnI measurements and cardiac MRI results.
COMPARISON WITH HISTORICAL TRANSCRANIAL DOPPLER DATA
The HITS observed by ICD were compared to cerebral HITS using our own historical transcranial Doppler data6.
RAPID PACING INDUCED HYPOTENSION AND BLOOD PRESSURE RECOVERY TIME
The overall duration of rapid pacing and the overall time of subsequent blood pressure recovery to pre-pacing values were measured.
TROPONIN I
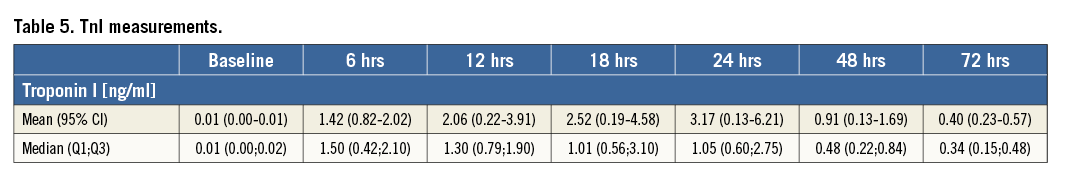
Venous blood samples were drawn before the procedure and at six, 12, 18, 24, 48, and 72 hours, and TnI was measured by a specific two-sided immunoassay (Dimension Xpand®; Siemens Healthcare, Erlangen, Germany). To assess the extent of peri-interventional myocardial injury, the area under the curve (AUC) for TnI serum concentration over 72 hours after TAVI was calculated according to the trapezoidal rule.
POST-PROCEDURAL PVLs
Post-procedural PVLs were assessed angiographically, echocardiographically and haemodynamically8.
CARDIAC MRI
Cardiac MRI examinations were performed pre- and post-interventionally on a 3 Tesla MR scanner (MAGNETOM® Skyra; Siemens Healthcare) including the acquisition of late gadolinium enhancement (LGE) images using gadobutrol (Gadovist®; Bayer Healthcare, Leverkusen, Germany). Segmented two-dimensional inversion-recovery turbo fast low-angle shot sequences were acquired 10 minutes after contrast agent injection (TE 1.56 ms, TR 6.46 ms, FA 20°, matrix 256×173, slice thickness 8 mm, bandwidth 465 Hz/pixel) with electrocardiographical triggering and in breath-hold.
STATISTICS
Data are expressed as mean and standard deviation, and categorical data as percent. Continuous variables are expressed as mean and 95% confidence intervals (CI) throughout the text; medians and lower (Q1) and upper (Q3) quartiles are additionally presented in the Tables. Continuous variables were compared with the t-test for dependent and independent samples. Spearman’s coefficient was used to quantify correlations. Variables were reviewed for clinical significance before testing. Univariate and multivariate regression analyses were performed to detect variables associated with increased periprocedural HITS. A p-value <0.05 was considered statistically significant. All statistical analyses were performed with the use of the SPSS software package, Version 21.0 (IBM Corp., Armonk, NY, USA). The authors had full access to and take full responsibility for the integrity of the data. All authors have read and agree to the manuscript as written.
Results
PATIENT CHARACTERISTICS
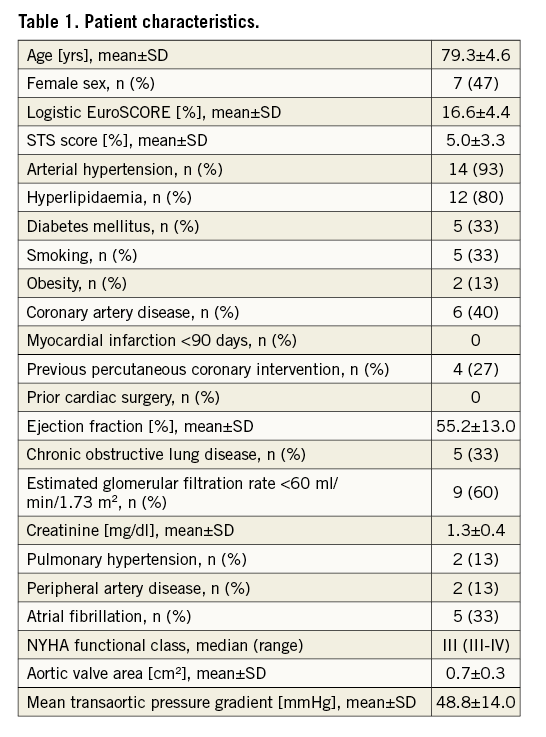
Our study cohort represents a typical TAVI population (Table 1).
PROCEDURAL RESULTS
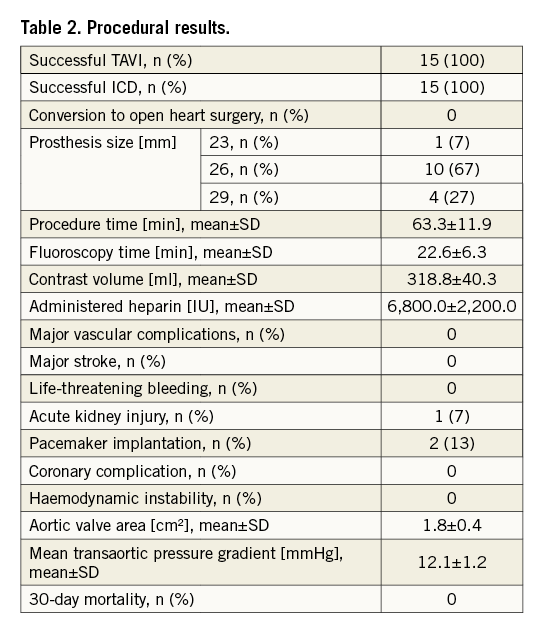
Transfemoral TAVI was technically successful in all patients without any deaths within 30 days (Table 2). In two cases (12%), a post-procedural pacemaker implantation was necessary due to a third-degree atrioventricular block precluding a post-interventional MRI.
INTRACORONARY DOPPLER
ICD was uneventful in all patients without any adverse events.
CORONARY FLOW DYNAMICS
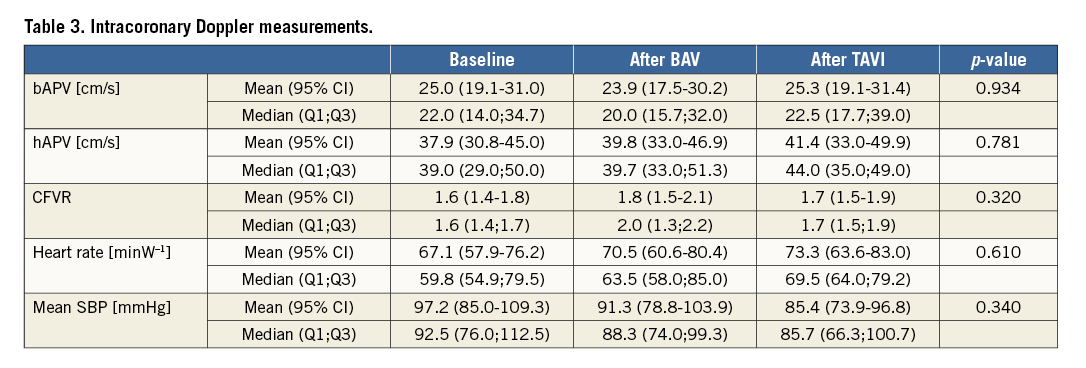
CFVR was impaired in all patients at baseline (compared to expected normal values >2.5-3.0), and there was no significant increase after valvuloplasty nor after TAVI. Likewise, there was no significant increase in hAPV. bAPV was increased at baseline (compared to expected normal values of 10-15 cm/s) and did not change significantly after valvuloplasty or after TAVI (Table 3).
HITS
During TAVI, intracoronary HITS were observed in all cases and during every defined procedural step (Table 4). The highest numbers of HITS were observed during the initial crossing of the native valve and during the positioning of the bioprosthesis. The other procedural steps were associated with fewer HITS. Of note, a higher number of HITS was observed during preparatory valvuloplasty than during deployment of the bioprosthesis.
Interobserver agreement of the HITS count among the three blinded observers had a mean difference of 3%.
Acknowledging the different duration of the procedural steps, we also calculated the number of HITS/s for each step (Table 4). As expected, the highest amount of HITS/s was observed during positioning of the bioprosthesis. In contrast, fewer HITS/s were detected during initial valve crossing, valvuloplasty and valve implantation. Nevertheless, the numbers of HITS/s during those steps were still higher than during the other procedural steps.
Overall, intracoronary HITS were predominantly observed during diastole except for the initial wire crossing of the aortic valve where 69% of HITS occurred in systole.
No predictors for increased procedural HITS could be derived from univariate and multivariate regression analysis, including the European System for Cardiac Operative Risk Evaluation (EuroSCORE) score, Society of Thoracic Surgeons (STS) score, mean transaortic gradient and valve area at baseline.
There were no significant correlations between coronary flow dynamics, as well as their intraprocedural changes, and the amount of HITS.
COMPARISON WITH HISTORICAL TRANSCRANIAL DOPPLER DATA
Overall, the total number of HITS was lower than in our historical transcranial Doppler cohort (232 [146-317] versus 482 [395-597]; p<0.001) (Figure 2), but HITS were similarly distributed with high numbers of transcranial and intracoronary HITS during valve positioning. However, initial crossing of the native calcified valve was associated with significantly more HITS on intracoronary than on transcranial Doppler (66 [32-100] versus 28 [17-38]; p<0.05) and even represented the step with the highest number of HITS in the ICD cohort.
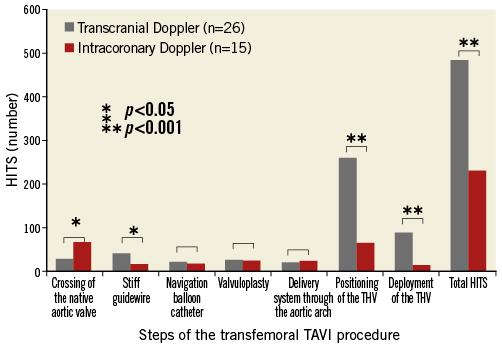
Figure 2. Intracoronary Doppler results. Comparison of intracoronary Doppler HITS during transfemoral TAVI with transcranial Doppler HITS (historical data).
RAPID PACING INDUCED HYPOTENSION AND BLOOD PRESSURE RECOVERY TIME
The overall duration of rapid pacing was 33.0±8.0 s and the time of blood pressure recovery to pre-pacing values was 47.3±36.1 s, the latter not being influenced by vasoactive drugs administered prior to rapid pacing (maximum catecholamine concentration <0.025 µg/kg/min).
TROPONIN I
All baseline serum TnI concentrations were lower than 0.1 ng/mL and, in all patients, serum TnI concentrations increased after TAVI (Table 5). TnI peaked at a median of 24 hours (18;24) after the procedure to 3.3 (0.5-6.1) ng/ml, and TnI AUC over 72 hours was 104 (21-187) ng/ml.
There was no correlation between the amount of HITS and AUC TnI over 72 hours. There was also no significant correlation between CFVR as an indicator for procedural microembolisation and AUC TnI. However, there were positive correlations between the overall duration of rapid right ventricular pacing (r=0.596, p=0.025) as well as the time of blood pressure recovery (r=0.636, p=0.010) (Figure 3) and AUC TnI, suggesting hypotension-induced ischaemia as a potential cause for myocardial injury.
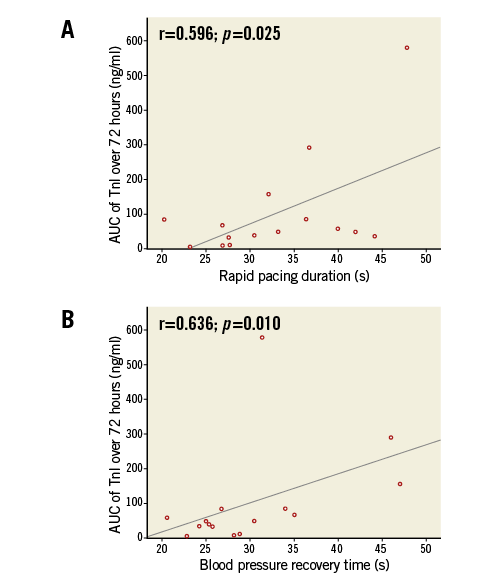
Figure 3. Hypotension-induced myocardial injury after TAVI. Correlations between rapid pacing duration (A) as well as blood pressure recovery time (B) and AUC TnI, suggesting hypotension-induced ischaemia as a potential cause for myocardial injury.
POST-PROCEDURAL PVLs
There was only one patient with more than mild PVL after TAVI and consequently no association with myocardial injury.
CARDIAC MRI
Pre-procedural cardiac MRI revealed no pathological findings, and in only a single case was a small mid-myocardial LGE in the lateral wall discovered post-procedurally. Consequently, no correlation was found between LGE and HITS or the TnI AUC over the first 72 hours.
Discussion
This hypothesis-generating study provides novel mechanistic insights into the origin of myocardial injury after transfemoral, balloon-expandable TAVI. It appears that post-procedural increases of TnI are related rather to periprocedural hypotension with hypoperfusion-induced diffuse ischaemia than to periprocedural microembolisation, despite the presence of embolic HITS in all patients, or to post-procedural PVLs.
In concordance with previous studies1-4, transfemoral TAVI was associated with myocardial injury, reflected by post-interventional increases in TnI, in all patients. As expected, ICD monitoring revealed HITS during all procedural steps, with the highest number of HITS during initial valve passage and valve positioning, reminiscent of what has recently been described for cerebral embolisation but with a lower amount of intracoronary compared to cerebral HITS6. Measurements of HITS in only one of the three major coronary vessels, the relatively small calibre of the coronary ostia compared to the larger diameter supra-aortic vessels and the fact that coronary perfusion mainly occurs during diastole may explain this observation. Yet, the temporal occurrence of coronary HITS was comparable to that of cerebral HITS, except for a higher number of coronary HITS during the initial wire crossing of the aortic valve. Interestingly, those intracoronary HITS occurred mainly during systole. This finding may be speculatively explained by two facts, namely that the native leaflets approximate the coronary ostia during systole and that the operator aims to cross the valve with the wire in an open state. Hence, most manipulation supposedly occurs during systole.
Coronary microembolisation has already been extensively studied during percutaneous coronary interventions (PCI) using ICD surveillance15-17, but has also been shown to occur in an experimental model of aortic valvuloplasty18. In the setting of PCI, transient TnI increases are common, associated with a worse clinical outcome19-21, and they correlate with the number of coronary microemboli assessed by ICD15,22,23. Cardiac MRI also often shows LGE after PCI as evidence of myocardial injury, and LGE again correlates with the TnI increase24. In our TAVI study, however, TnI AUC over 72 hours did not correlate with the number of HITS measured by ICD, and we only observed a small, localised mid-myocardial LGE in a single patient.
The lack of association between TnI increase and intracoronary HITS in our study is intriguing. Obviously, there is a discrepancy between the great amount of detected intracoronary HITS as a surrogate for coronary microembolisation and both the low degree of myocardial injury and the lack of adverse clinical cardiac events. However, that discrepancy is also observed for the brain. Cerebral diffusion-weighted MRI has revealed a great amount of new, clinical silent foci of restricted diffusion after TAVI, contrasting with only few clinically apparent neurological events25,26, and transcranial Doppler has also shown omnipresent periprocedural HITS without overt clinical effect6,27. Interestingly, we did not observe any relevant procedural changes in coronary flow dynamics before and after TAVI, corresponding to what has recently been described by Camuglia et al with the Medtronic CoreValve® (Medtronic, Minneapolis, MN, USA) prosthesis28, and there was no correlation between coronary flow dynamics and TnI AUC or the amount of HITS. Although this analysis needs to take into account the small sample size of our study, it appears that procedural embolisation may not be the main cause of myocardial injury in the setting of TAVI.
In this context, the discrepancy between serum (TnI) and imaging markers (LGE) of myocardial injury is of further interest. Similar to our finding, Biere et al recently reported that transapical TAVI was only associated with small apical infarctions related to left ventricular access without any functional impairment29, and no other LGE was observed, not even in the periprosthetic area, thus also excluding direct tissue trauma as a cause for myocardial injury. In another cardiac MRI study including 61 patients, Kim et al observed LGE in 20% of transfemorally treated patients. Although the prevalence of LGE was higher than in our study, possibly due to a sicker patient population, the authors also noted a lack of correlation between the level of cardiac biomarkers, specifically high-sensitive troponin T and CK-MB, which were also increased after TAVI in all patients, and the mass of new LGE4. In their study, an impaired renal function was the only parameter associated with new-onset LGE, potentially indicating a higher susceptibility to myocardial injury as known from PCI7. The limited accuracy of MRI in the detection of LGE in vivo may offer one explanation for this descrepancy30. Of note, non-localised, global ischaemia cannot be identified by LGE. Consequently, “this discrepancy argues against an embolic-only explanation for the universally noted myocardial injury”7.
However, we observed positive correlations between the overall duration of rapid right ventricular pacing as well as the time of blood pressure recovery and AUC TnI, suggesting hypotension-induced ischaemia as a contributing factor for the most likely multifactorial myocardial injury after TAVI. Clinically, this finding suggests the omission of preparatory balloon aortic valvuloplasty and short rapid pacing periods as simple, first-line strategies to reduce myocardial injury associated with transfemoral TAVI. Whether these preventive strategies are effective and improve outcome warrants further study, but may be expected: prolonged and persistent post-procedural hypotension not related to hypovolaemia is associated with increased in-hospital complications, mainly minor strokes, and long-term death in the setting of carotid artery intervention using balloon-expandable stents31.
Lastly, with more than mild PVL present only in a single patient, this study could not find an association between myocardial injury and PVL severity. Nevertheless, a previous, larger-scale study revealed higher TnI concentrations in patients with haemodynamically relevant PVLs, this finding linking PVL severity to ischaemia and underlining the need to reduce their incidence and severity8.
Study strengths and limitations
Our study is only descriptive and hypothesis-generating, but despite its size, which was small due to the advanced complexity of the TAVI procedure, reflects typical TAVI patients treated with a balloon-expandable bioprosthesis. However, no comparison was performed with a self-expanding device, since continuous Doppler monitoring throughout CoreValve implantation was deemed prohibitive due to the risk of rupture of the jailed Doppler wire during retrieval after valve implantation. Similarly, flow measurements and monitoring in all three major coronary vessels, and not only the LAD, were considered an excessive risk. Also, no comparison was performed with transapical TAVI since this procedure includes left ventricular trauma by apical access causing a TnI increase unrelated to coronary microembolisation and thereby probably diluting any analysis. While ICD can assess coronary microemboli as HITS and identify their appearance in every single procedural step of the transfemoral TAVI procedure, characterisation of individual emboli is impossible by ICD since this method does not distinguish between air microbubbles and solid material. Therefore, episodes of contrast injections with a likelihood of air emboli were excluded from the present analysis. Also, the size of an individual embolus cannot be derived from its signal intensity in the Doppler spectrum, but may determine its clinical consequences32. Fortunately, coronary artery obstruction by large emboli is a rare event and apparently did not occur in our study.
Conclusion
The current study demonstrates that coronary microembolisation occurs during each procedural step of transfemoral, balloon-expandable TAVI. However, no association was found between the amount of HITS and perioperative TnI serum concentrations or LGE on cardiac MRI, but there was an association between the overall duration of rapid right ventricular pacing as well as the time of subsequent blood pressure recovery and TnI. Whilst most likely multifactorial, myocardial injury after TAVI thus appears to be more related to periprocedural hypotension with hypoperfusion-induced diffuse ischaemia than to periprocedural microembolisation despite the presence of embolic HITS in all patients.
| Impact on daily practice Coronary microembolisation appears to be a TAVI-inherent phenomenon, but not the main cause for the most likely multifactorial myocardial injury associated with the procedure. Our study suggests periprocedural hypotension with hypoperfusion-induced diffuse ischaemia as being an important contributing factor. This finding suggests that the omission of preparatory balloon aortic valvuloplasty and short rapid right ventricular pacing periods are simple, first-line strategies to reduce myocardial injury during TAVI. |
Acknowledgements
We thank Nils Lehmann from the Institute of Medical Informatics, Biometry and Epidemiology of the University Hospital Essen for his advice.
Conflict of interest statement
P. Kahlert, D. Wendt and M. Thielmann have received honoraria as clinical proctors for Edwards Lifesciences Inc. The other authors have no conflicts of interest to declare.

