
Transcatheter aortic valve implantation (TAVI) is now well established as an alternative to aortic valve replacement (AVR) in patients who are considered high-risk for surgery1, or who are inoperable2. Subsequent data have underscored the longevity of TAVI3, and the procedure has become so successful that trials are now ongoing (e.g., SURTAVI) to examine its potential use in an intermediate-risk population. Cardiovascular imaging techniques, particularly echocardiography and CT, play a vital role in TAVI4. However, the literature examining the role of cardiovascular magnetic resonance (CMR) in TAVI is currently limited to only a handful of papers.
In this edition of EuroIntervention, Ribeiro et al5 describe their findings using CMR to look for myocardial injury in patients who had recently undergone TAVI. In a group of 37 patients, only one of whom did not show a post-procedural rise in troponin, the authors found that the only new areas of gadolinium enhancement seen were at the ventricular apex in patients who had undergone a transapical (TA) TAVI; this is similar to the apical scar often seen following insertion of a left ventricular assist device6 (Figure 1). A TA approach involves the insertion of a large calibre (≥18 Fr) sheath into the ventricular apex, and some data have shown an increased mortality risk with this approach7. Although this may largely be driven by higher rates of bleeding and stroke7, the results of the current paper would suggest that some of the risk of the transapical approach may be due to the long-term myocardial injury caused; TA patients were noted to have lower left ventricular ejection fraction at follow-up.
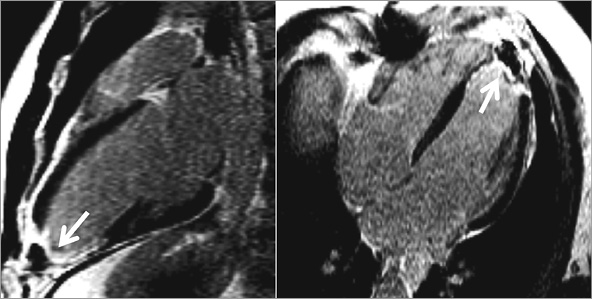
Figure 1. Example of transmural apical scarring (arrows) following left ventricular assist device insertion depicted on the late gadolinium enhancement images acquired in the vertical and horizontal long-axis views of the left ventricle (from Moon et al 2003), similar to that seen in the study by Ribeiro5. Reproduced in accordance with BioMed Central’s Open Access Charter.
The lack of gadolinium enhancement seen in any other myocardial areas is an encouraging finding given that even minor necrosis of the order of 1.4% of ventricular myocardium can be detected with CMR. However, this finding must be contrasted with the results of MRI studies of the brain, which have shown that the vast majority of TAVI patients suffer some form of cerebral microembolic damage8. Therefore, given the large volume of embolic debris released during TAVI, some passage of this material down the coronary arteries –leading to post-procedural myocardial necrosis beyond the resolution of CMR– remains a possibility9. Indeed, previous studies that have performed CMR following PCI have shown that even relatively large embolic loads (coronary plaques up to 50 mm3 in volume) may be treated without causing any subsequent myocardial enhancement10.
In addition, many patients with severe aortic stenosis have significant left ventricular hypertrophy which predisposes to subendocardial myocardial ischaemia11, particularly during placement of the TAVI prosthesis using a period of rapid ventricular pacing: a recent publication by Selle et al12 has demonstrated that even short periods of rapid ventricular pacing can lead to microcirculatory arrest. This propensity to subendocardial ischaemia may explain why Yong et al found that a longer procedural duration and the absence of beta-blockade were associated with myocardial injury13. Therefore, although a lack of myocardial gadolinium enhancement on post-TAVI CMR may be reassuring, this does not mean that no permanent myocardial injury has taken place. Newer CMR imaging techniques, such as T1 mapping, may reveal global diffuse myocardial interstitial fibrosis that is not currently visualised using late gadolinium enhancement14.
A couple of limitations of the study must be highlighted, not least the small numbers of patients studied. A larger study, encompassing patients with and without significant left ventricular hypertrophy and perhaps confined to transfemoral cases only, would help to clarify the true incidence of CMR-detected myocardial injury following TAVI. Such a study must include not only T1 mapping but also T2-weighted myocardial oedema imaging sequences; the latter were not used in the current study but have widely been considered to display “area at risk” in CMR studies following myocardial infarction15.
What is the role of CMR in TAVI?
Looking forward, the use of CMR in patients undergoing, or being considered for, TAVI is likely to expand. Prior to TAVI, MRI can provide several important pieces of structural information: in addition to precise quantification of ventricular volumes and function, aortic valve planimetry can be performed to determine valve area accurately, and late gadolinium enhancement can show areas of myocardial scarring. Furthermore, work from our institution has shown that MRI can provide accurate aortic annulus measurements which, in terms of predicting the presence and severity of aortic regurgitation after TAVI, are comparable to those obtained using CT16. Also, in cases where CT angiography is not available or is deemed inappropriate, magnetic resonance angiography of the peripheral vasculature can be performed to assess access routes.
Following TAVI, CMR can be used to provide an accurate estimate of ventricular function, and a recent publication has also shown the accuracy of CMR to detect and quantify paravalvular leak17. Ultimately, however, unless any post-procedural CMR-specific measurements can be shown to lead to treatment changes that affect prognosis, it will remain highly debatable whether it is worth the time and expense to perform CMR on a routine basis after TAVI; echocardiography may be sufficient for the majority of cases.
In the longer term, however, a role may arise for percutaneous valve interventions such as TAVI to be completely MRI guided; real-time transarterial implantation of the Medtronic CoreValve (Medtronic, Minneapolis, MN, USA), using a modified, MRI-compatible delivery device, has already been described in a swine model18.
In summary, although larger studies are needed, the paper by Ribeiro et al reassures us that contemporary TAVI practice, outside of TA procedures, generally causes little significant macroscopic myocardial injury. As the volume of TAVI procedures being performed year-on-year continues to grow, and its application is extended to younger patients, it is likely that larger CMR series will be reported, further emphasising its importance as an imaging tool in the diagnosis and management of structural heart disease.
Conflict of interest statement
The authors have no conflicts of interest to declare.

