
Abstract
Aims: Myocardial injury assessed using cardiac biomarker release is ubiquitous following surgical aortic valve replacement (SAVR) and transcatheter aortic valve implantation (TAVI), preventing accurate discrimination between focal myocardial infarction (MI) and global injury. Cardiovascular magnetic resonance (CMR) late gadolinium enhancement (LGE) imaging was used to compare rates of new MI following SAVR and TAVI.
Methods and results: Identical CMR scans were obtained at baseline and six months post procedure in ninety-six patients undergoing SAVR (n=39) and TAVI (n=57). The rate of new MI was greater following SAVR than TAVI (SAVR, n=10 [26%] vs. TAVI, n=3 [5%], p=0.004). Infarct mass was similar between groups (SAVR 1.1±0.6 vs. TAVI 2.0±1.4 g, p=0.395). New MI did not impact on change in LV ejection fraction (SAVR:LGE[+]2.2±4.7 vs. LGE[–]0.9±8.0%, p=0.437, TAVI:LGE[+]–0.9±6.0 vs. LGE[–]2.0±7.8%, p=0.420). Thirty-four patients (60%) in the TAVI group had non-revascularised coronary artery disease (CAD) at the time of TAVI, of whom three (9%) had new MI.
Conclusions: MI is an infrequent complication of TAVI but is more common following SAVR. Infarct size is small following both procedures. The low new infarct rate in TAVI, especially in the context of high rates of non-revascularised CAD, strengthens data from previous studies suggesting that coronary revascularisation pre-TAVI may be unnecessary.
Abbreviations
AF: atrial fibrillation
AS: aortic stenosis
CABG: coronary artery bypass grafting
CAD: coronary artery disease
CMR: cardiovascular magnetic resonance
IQR: interquartile range
LGE: late gadolinium enhancement
LV: left ventricular
LVEF: left ventricular ejection fraction
MI: myocardial infarction
SAVR: surgical aortic valve replacement
TAVI: transcatheter aortic valve implantation
VARC: Valve Academic Research Consortium
Introduction
Surgical aortic valve replacement (SAVR) remains the recommended technique for those with severe symptomatic aortic stenosis (AS); however, transcatheter aortic valve implantation (TAVI) is now an alternative for those at high surgical risk1. Myocardial injury assessed using cardiac biomarker release is associated with an adverse outcome following both cardiac surgery2 and transcatheter intervention3. Mechanisms are varied and are depicted in Figure 1. Cardiac biomarker release is ubiquitous following both procedures4,5, preventing accurate discrimination between release due to focal myocardial infarction (MI) and global/diffuse myocardial injury. Furthermore, the importance of coronary artery disease (CAD) and the completeness of revascularisation prior to TAVI are debated6. Cardiovascular magnetic resonance (CMR) imaging is the reference standard imaging test to evaluate the incidence of post-procedural MI using the late gadolinium enhancement (LGE) technique and can detect even tiny areas of focal scar7. Our study aimed to compare rates of new MI, using CMR LGE imaging before and six months following SAVR and TAVI.
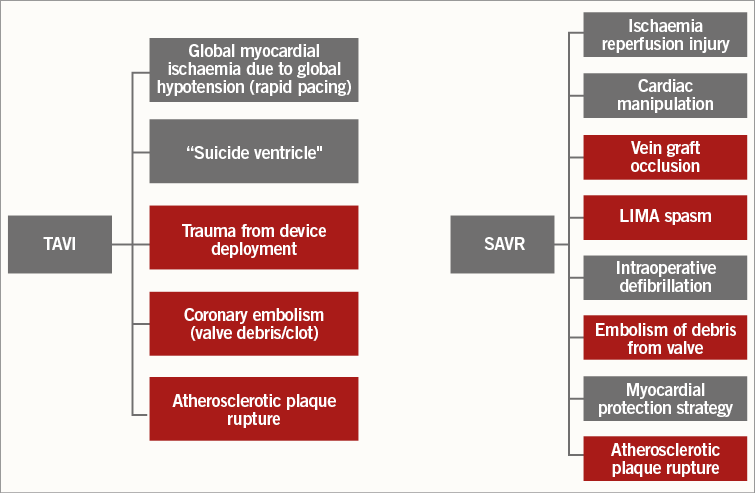
Figure 1. Potential mechanisms for myocardial injury following TAVI and SAVR. All mechanisms of myocardial injury can lead to a cardiac biomarker release. Mechanisms which can lead to focal MI and can be assessed using CMR LGE are shown in red. LIMA: left internal mammary artery
Methods
Between January 2009 and April 2014, 130 patients with severe AS undergoing either SAVR or TAVI with or without concomitant coronary artery bypass grafting (CABG), at a single tertiary centre (Leeds General Infirmary, Leeds, UK) were prospectively recruited. Severe AS was defined as an echocardiographically derived aortic valve area of ≤1.0 cm2, peak aortic velocity of >4 m/sec or mean pressure gradient of >40 mmHg8. Decision for aortic valve intervention was made by a dedicated Heart Team in accordance with international guidelines1. The presence of significant CAD was determined by the occurrence of >50% stenosis by visual estimation in any major epicardial vessel (>2.5 mm diameter) on a preprocedural coronary angiogram. Patients with contraindications to contrast CMR were excluded. All patients provided written informed consent. The study was approved by the institutional ethics committee and complied with the Declaration of Helsinki.
AORTIC VALVE INTERVENTION
SAVR was performed using a standard technique on cardiopulmonary bypass via a midline sternotomy incision and mild systemic hypothermia (30-34°C). Systemic heparinisation with standard aorto-right atrial cannulation was used to establish cardiopulmonary bypass. Cold blood cardioplegic arrest of the heart and pericardial carbon dioxide were used in all cases. The aorta was cross-clamped and an aortotomy performed with the size and type of prosthesis selected according to annulus size, patient characteristics, surgical and patient preference. Concomitant CABG was performed using a combination of left internal mammary artery and saphenous vein grafting to significantly diseased major vessels with the aim of complete revascularisation in all patients. Patients were discharged on aspirin monotherapy or warfarin monotherapy in the case of mechanical valve implantation or atrial fibrillation (AF).
TAVI was performed using the self-expanding CoreValve®/Engager™ devices (Medtronic, Minneapolis, MN, USA) or the mechanically expanded Lotus™ valve (Boston Scientific Corp., Marlborough, MA, USA) by two experienced, high-volume operators. The transfemoral approach was preferred but other approaches were used in the case of unsuitable femoral access. Balloon valvuloplasty was performed before device deployment in most cases and patients typically underwent two to three bouts of rapid pacing. All patients received heparin to achieve and maintain an activated clotting time of >250 sec. Dual antiplatelet therapy (aspirin 75 mg/day and clopidogrel 75 mg/day) was administered for three to six months post procedure with aspirin monotherapy thereafter, or warfarin monotherapy in the case of AF.
CMR PROTOCOL
Identical CMR scans were obtained on the same imaging platform at baseline (median one day pre-procedure, interquartile range [IQR] 14 days) and at a median of six months (IQR one month) following aortic valve intervention using the same 1.5T scanner (Intera 1.5T™; Philips Healthcare, Eindhoven, the Netherlands, or Avanto; Siemens Medical Systems, Munich, Germany). Multislice, multiphase cine imaging was performed using a standard steady-state free precession pulse sequence in the short axis (10 mm thickness, no interslice gap, 30 phases) to cover both ventricles. Standard 2D short-axis LGE imaging using an inversion recovery-prepared T1-weighted gradient echo pulse sequence (10-12 short-axis slices, 10 mm thickness, matrix 240×240, typical field of view 340 mm, SENSE factor 1.6) was performed following a Look-Locker pulse sequence, 10-15 minutes after the administration of 0.2 mmol/kg of gadoteric acid (Dotarem®; Guerbet, Villepinte, France). Phase-swap and cross-cut imaging was performed where necessary.
CMR ANALYSIS
CMR analysis was performed by a single operator (L.E. Dobson). Endocardial and epicardial left ventricular (LV) borders were manually contoured at end-diastole and end-systole to allow the calculation of volume (summation of discs methodology) and mass (epicardial volume–endocardial volume multiplied by myocardial density [1.05 g/cm3]). Values were indexed to body surface area. For analysis of the LGE images, each slice was visually inspected for the presence or absence of LGE by two operators independently blinded to clinical and procedural data. Any discrepancy between operators was reviewed by a third operator to reach a consensus decision. Presence of new LGE was determined by direct comparison of pre- and post-procedure scans. The location and transmural extent of LGE according to the 17-segment American Heart Association model was recorded. Quantification of MI was performed using computer-assisted planimetry (cmr42; Circle Cardiovascular Imaging, Calgary, Canada). Infarct mass was expressed in g of tissue and as a percentage of overall LV mass.
STATISTICAL ANALYSIS
All statistical analyses were performed using the PASW software package, Version 21 (IBM Corp., Armonk, NY, USA). Data are presented as mean±SD, median (IQR) or frequency (percentage). After testing for normality (Shapiro-Wilk test), differences between means were evaluated using the Student’s t-test for normally distributed data and the Mann-Whitney test for non-parametric data. The chi-squared test was used for comparing categorical variables. A two-sided p<0.05 was considered statistically significant.
Results
PATIENT DEMOGRAPHIC, PROCEDURAL AND CLINICAL DATA
One hundred and thirty patients were recruited into the study. Ninety-six patients undergoing either SAVR (n=39) or TAVI (n=57) completed both baseline and six-month post-procedure CMR scans (Figure 2). No patients had a hospital admission with acute coronary syndrome or underwent any revascularisation procedure between hospital discharge and the six-month follow-up scan. Baseline data can be seen in Table 1.
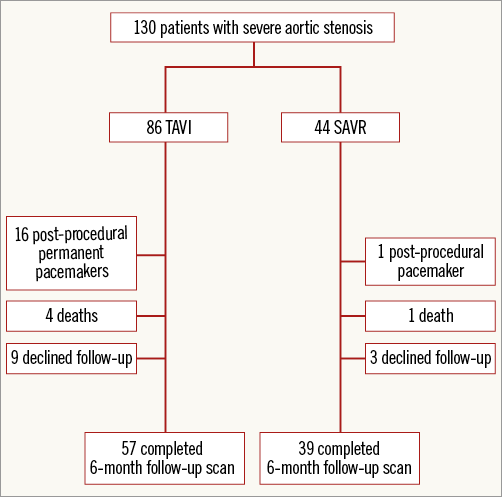
Figure 2. Patient recruitment pathway.
Concurrent CABG was performed in 16 (41%) of the SAVR patients. The mean cross-clamp time and cardiopulmonary bypass time were 79±38 and 108±45 min, respectively. The majority of SAVR patients underwent bioprosthetic (n=34, 87%) valve replacement. In the TAVI group, access was most commonly via the femoral route (n=49, 86%) and the majority of implants were CoreValves (n=45, 79%).
BASELINE LATE GADOLINIUM ENHANCEMENT IMAGING
LGE data pre- and post-TAVI and SAVR were available for all analysed patients. Twenty-four (42%) patients in the TAVI group had infarct pattern LGE at baseline with an average mass of 14.2±10.4 g or 10±7.9% of total LV mass. Of these, only seven (29%) had a clinical history of MI, with a further eight (30%), 13 (54%) and 10 (42%) having a history of percutaneous coronary intervention, CABG and AF, respectively. Nine (23%) SAVR patients had infarct pattern LGE at baseline with a mean mass of 19.7±14.3 g or 11.3±6.9% of total LV mass. Of these, only two (22%) had a history of MI and a further single (11%) patient had a history of AF.
NEW INFARCT RATE FOLLOWING SAVR AND TAVI
The rate of new MI defined by LGE (LGE[+]) was greater in the SAVR group than in the TAVI group (SAVR, n=10 [26%] vs. TAVI, n=3 [5%], p=0.004). Absolute mean infarct mass was similar between the two groups (SAVR 1.1±0.6 vs. TAVI 2.0±1.4 g, p=0.395), as was infarct mass as a percentage of LV mass (SAVR 1.0±0.4 vs. TAVI 2.2±1.3%, p=0.268). None of the new MI cases occurred in a previously infarcted territory. Details of individual new MI patients can be seen in Figure 3. Baseline and six-month cardiac parameters according to LGE status are shown in Table 2.
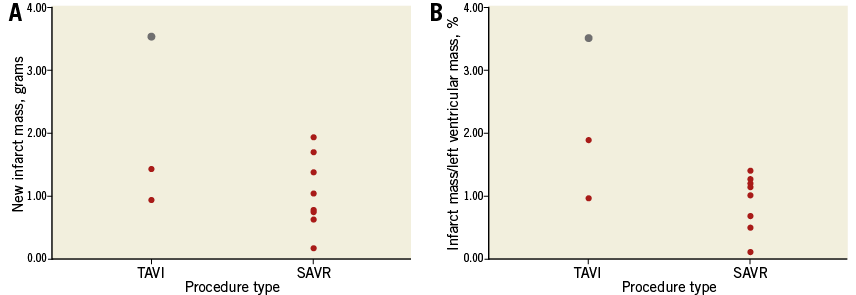
Figure 3. New infarct mass expressed in absolute terms and as a percentage of left ventricular mass according to procedure type. The red dots represent individual patients and the grey dot represents the only clinically detected MI according to VARC criteria.
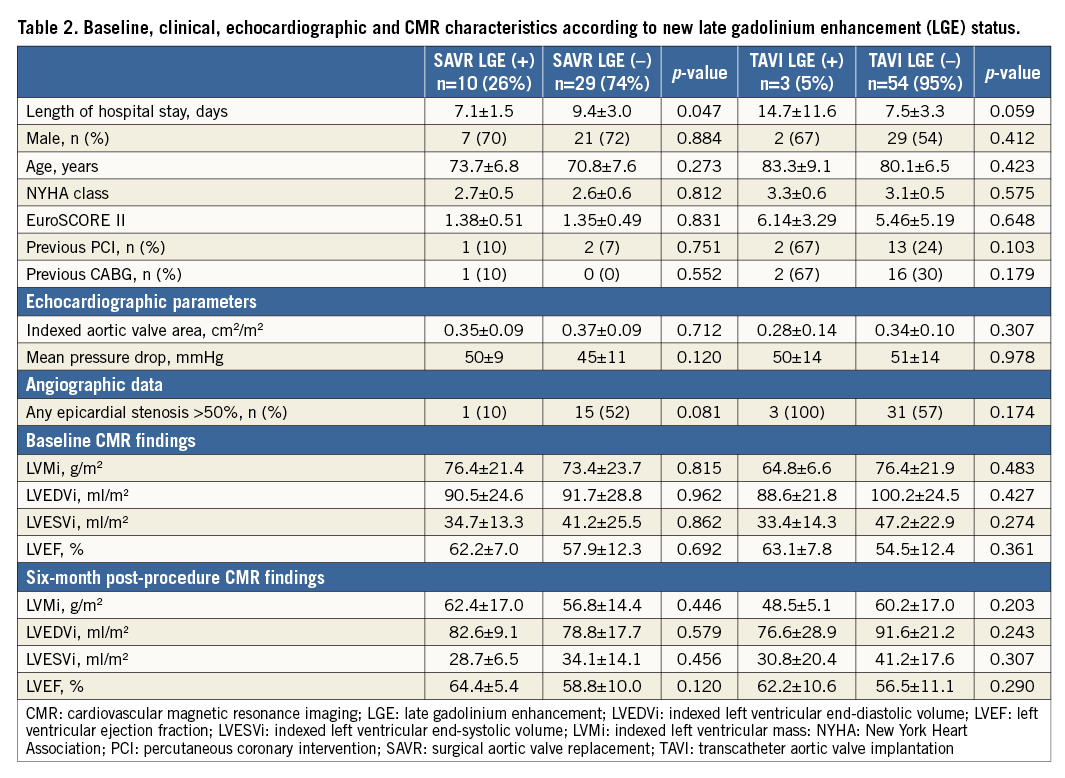
TAVI
There were three new infarcts in the TAVI population (Table 3), all of whom had significant pre-existing CAD. One patient underwent simultaneous PCI during the TAVI procedure. Only one TAVI patient had a clinically detectable post-procedural MI according to Valve Academic Research Consortium (VARC) criteria9 (Figure 3, Figure 4). Change in LV ejection fraction (LVEF) according to new MI status was similar between groups (LGE[+]-0.9±6.0 vs. LGE[–]2.0±7.8%, p=0.420). Valve size (LGE[+]27±4 vs. LGE[–] 28±2 mm, p=0.933), procedure time (LGE[+]135±23 vs. LGE[–] 161±51 min, p=0.511) and valvuloplasty rate (LGE[+]100% vs. LGE[–]91%, p=0.581) were similar according to new MI status.
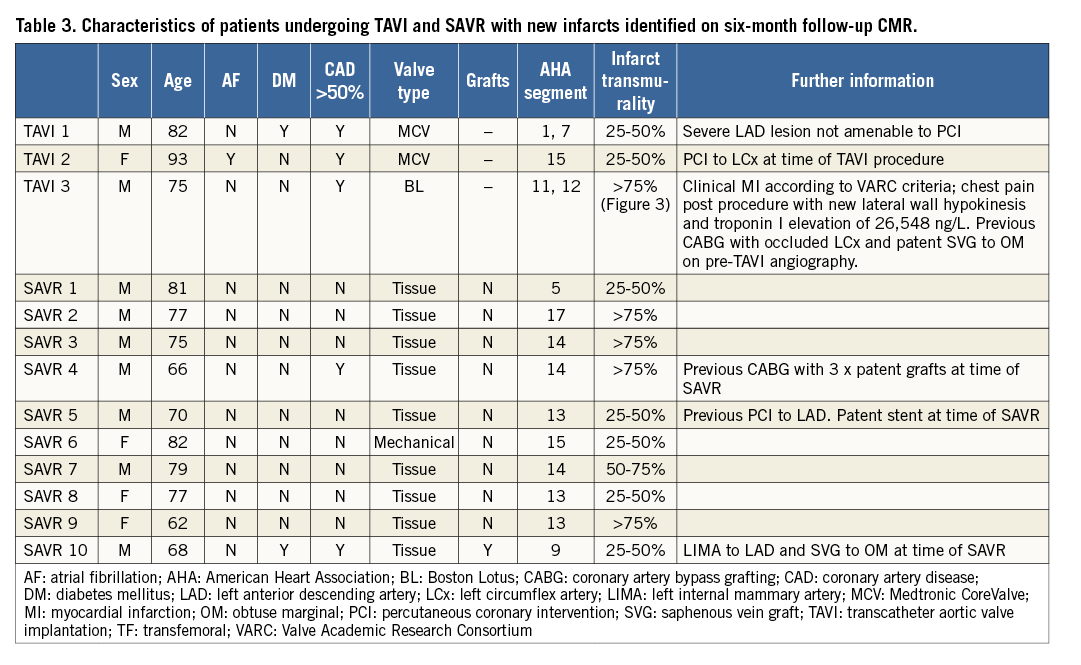
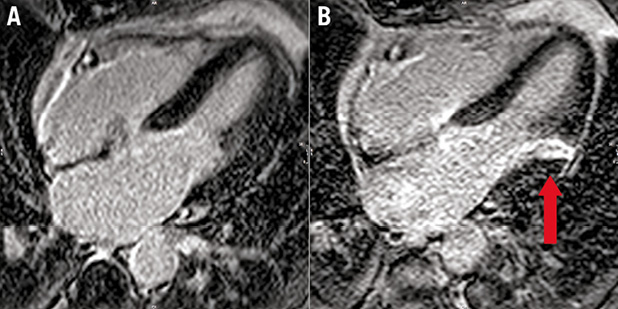
Figure 4. Example of new MI demonstrated using CMR LGE imaging. A) A horizontal long-axis image of the left ventricle prior to TAVI. The septal and lateral left ventricular walls can be seen and there is no evidence of scar, as shown by the uniform dark appearance of the myocardium. B) The same patient six months following TAVI with an area of transmural hyperenhancement (LGE) indicative of MI (arrow).
SAVR
There were 10 new infarcts in the SAVR population, only one of which had significant CAD and was revascularised at the time of surgery (Table 2). Individual SAVR patient characteristics of those with new LGE confirmed MI are shown in Table 3. None of the SAVR LGE(+) events was detected clinically. Patients undergoing CABG were less likely to have a new MI than those not requiring concurrent revascularisation (CABG 6% vs. no CABG 39%, p=0.021). There was no difference in change in LVEF according to new LGE status (LGE[+]2.2±4.7 vs. LGE[–]0.90±8.0%, p=0.437). Mean cardiopulmonary bypass time (LGE[+]88.5±31.1 vs. LGE[–]114.5±47.4 min, p=0.112) and aortic cross-clamp time (LGE[+]66±25 vs. LGE[–]84±42 min, p=0.164) were similar.
Discussion
This study is the first to demonstrate comparative post-procedural MI rates following TAVI and contemporary SAVR using CMR LGE imaging. We have shown that TAVI was associated with a significantly lower rate of post-procedural MI than SAVR, despite the TAVI population being older with more comorbidities. We have also demonstrated a low new MI rate in those undergoing TAVI with non-revascularised CAD, strengthening data from previous studies suggesting that TAVI may be safely performed without prior percutaneous coronary intervention3,10.
SAVR
To our knowledge, the new infarct rate using CMR LGE following SAVR has not been previously investigated. All of the infarcts were small with a mean overall mass of just over one gram and none was detected clinically. It is not surprising therefore that those with new MI had no significant deterioration in LVEF compared with those without. The current European Society of Cardiology guidelines suggest revascularisation of coronary artery stenoses of ≥70% at the time of SAVR1; however, these recommendations are based on evidence from registry data. In the light of the fact that in our study most of the MIs occurred in patients without significant CAD, and new MI was infrequent in TAVI patients with non-revascularised CAD, a prospective, randomised study investigating the impact of concurrent revascularisation of bystander CAD at the time of SAVR in elderly patients may be warranted in order to provide a better evidence base for these guidelines.
TAVI
Only one patient (2%) in the TAVI group had a clinically detected MI which fulfilled VARC criteria. This finding is in keeping with the low rates of clinically detected periprocedural MI found in the PARTNER study11. Out of 34 patients undergoing TAVI with non-revascularised CAD at the time of the TAVI procedure, only three (9%) had new MI, one of which may have been a result of concomitant percutaneous revascularisation rather than due to the TAVI procedure itself. This study suggests that the risk of MI being precipitated by periods of global hypotension during TAVI in the context of coronary stenosis is low. Our findings are consistent with other studies questioning the need for pre-TAVI coronary revascularisation. Rodés-Cabau et al3 did not find any influence of the presence of prior CAD and preprocedural revascularisation completeness on myocardial injury following TAVI, and Masson et al10 found that the presence of CAD was not associated with an increased risk of adverse events.
Our new infarct rate of 5% in the TAVI arm was lower than the 18% suggested by a similar-sized study by Kim et al12. Almost half of the patients in their study underwent transapical TAVI and it is not clear from their methodology whether scar related to the access site was included in the analysis. Transapical TAVI is associated with a two to four times increased level of post-procedure troponin release compared with the transfemoral route3, and apical LGE has been found to be almost universal on CMR imaging following transapical TAVI7. Also, in this study patients received clopidogrel monotherapy; however, in our investigation dual antiplatelet therapy was commenced, which may have afforded additional embolic protection. Kim et al12 also reported a reduction in LVEF in those patients with new MI – this was not found in our study or by others4,13. The fact that a 1.8% loss of LV myocardium pertained to a 10% reduction in LVEF in their study is surprising and suggests that new LGE was a surrogate marker for other adverse procedure-related factors.
Our findings are supported by a recent study by Kahlert et al14. They investigated 15 patients undergoing transfemoral TAVI using a Doppler wire positioned in the left anterior descending artery for the entire TAVI procedure. They described microembolic coronary artery showers at all stages of the procedure. On pre- and post-procedural CMR, only one patient had MI. There was no correlation between the number of coronary artery showers and troponin release.
CLINICAL CONTEXT
The impact of new infarct pattern LGE following aortic valve intervention is not yet known; in all but one case these infarcts were “silent” and of no obvious clinical relevance to the patient. Furthermore, the findings demonstrate that even when these “micro-infarcts” occur they are small. However, the presence of LGE following percutaneous coronary revascularisation has been linked to reduced survival15, and evidence of even a small amount of LGE (mean LV mass 1.4%) in patients with suspected CAD has been associated with a >7-fold risk of major adverse events16. Further studies are required to explore whether the presence of LGE following aortic valve intervention is associated with an adverse outcome. It is also worth noting that differing regimes of anticoagulation were used following TAVI and SAVR. It is possible that the TAVI dual antiplatelet therapy was protective against post-procedural embolic events. Differing anticoagulation regimens are currently under investigation.
Biomarker release is almost ubiquitous following aortic valve intervention due to the global insult to the ventricle (Figure 1). Barbash et al4 found elevated troponin in 98% of 150 patients following TAVI. The high sensitivity of cardiac biomarkers impedes their ability to detect focal MI following valve intervention13. Also, non-ischaemic ECG changes develop frequently following valve implantation17, meaning that the detection of true periprocedural MI is challenging. Thus, our study demonstrates the potential clinical utility of CMR LGE in the diagnosis of periprocedural MI.
Our findings also serve to reassure operators that TAVI is not associated with high rates of MI, even in the context of non-revascularised CAD, and that the strategy of proceeding to TAVI without prior percutaneous revascularisation may not expose the patient to excessive risk.
Limitations
As with all observational studies of SAVR and TAVI, the groups are not matched in terms of age, comorbidity or surgical risk, due to the current selection criteria for TAVI implantation. The death rate at six months following TAVI was more than double that of the SAVR population, which reflects the increased frailty of this patient population. We do not have autopsy data of these patients and therefore accept that this is a major limitation of this study. The high post-procedural permanent pacemaker rate following TAVI is a common limitation of all CMR-based TAVI studies12. Nonetheless, it is also a potential source of significant bias; however, 30-day and one-year rates of myocardial infarction were no different in the paced and non-paced population in a large registry study of over 2,500 patients involved in the PARTNER study17. Our follow-up scan was at six months following the procedure; therefore, it is difficult to be certain that the infarcts occurred at the time of the procedure and not due to an event in the months following the procedure, although none of our patients had an admission with chest pain or underwent coronary revascularisation in the time between hospital discharge and six-month follow-up. Our study did not include biomarker data, as it has been shown to show little relationship with MI in the post-procedural period; however, these data may have been helpful in delineating the timeline of the MIs observed.
Conclusions
Whilst MI assessed by CMR LGE is an infrequent complication of TAVI, it appears to be more common following SAVR. Absolute infarct size is small following both procedures and does not impact on post-procedural LVEF. The low new infarct rate in TAVI, especially in the context of high rates of non-revascularised CAD, is reassuring and strengthens data from previous studies suggesting that coronary revascularisation prior to TAVI may be unnecessary.
| Impact on daily practice The new MI rate following TAVI is low despite high rates of non-revascularised coronary artery disease at the time of the procedure, suggesting that pre-procedure revascularisation may not be warranted in some cases. CMR is better able to detect post-procedural MI than clinical assessment. The impact of these small post-procedural infarcts on clinical outcome remains to be established and further studies are required. |
Acknowledgements
The authors are grateful for the support of the research nurses and radiographers (Fiona Richards, Petra Bijsterveld, Lisa Clark, Gavin Bainbridge, Caroline Richmond and Margaret Saysell).
Funding
T.A. Musa is funded by a BHF Project Grant (PG/11/126/29321). P.P. Swoboda is funded by a BHF Clinical Fellowship (FS/12/88/29474). S. Plein is funded by a BHF Senior Research Fellowship (FS/10/62/28409). This study was part funded by the NIHR Leeds Clinical Research Facility. The views expressed are those of the author(s) and not necessarily those of the NHS, NIHR or the Department of Health.
Conflict of interest statement
J. Greenwood and S. Plein have received an educational research grant from Philips Healthcare. D. Blackman is a consultant and proctor for Medtronic and Boston Scientific. C. Malkin is a consultant and proctor for Boston Scientific. The other authors have no conflicts of interest to declare.

