Abstract
Cardiac computed tomography and magnetic resonance have important roles in patient selection, planning, and surveillance of various structural interventional procedures. The strengths of computed tomography (CT) are its superior spatial resolution, short acquisition time, and ability to view images in any plane using multiplanar reconstruction software. On the other hand, the advantage of magnetic resonance imaging (MRI) is the ability to provide combined haemodynamic and anatomical data, as well as tissue characterisation, without use of nephrotoxic contrast or radiation exposure. The choice of imaging modality is dependent on the specific structural heart intervention, patient-related factors, and institutional expertise.
Introduction
Both cardiac computed tomography and magnetic resonance have evolving roles in patient selection, planning, and surveillance of various structural interventional procedures. Each has its own unique strengths and weaknesses. Generally, the strengths of computed tomography (CT) are its superior spatial resolution, short acquisition time, and ability to view images in any plane using multiplanar reconstruction software. In addition, temporal resolution has increased with newer-generation CT scanners, allowing highly accurate assessment of cardiac volumes and function, as well as coronary stenosis evaluation. On the other hand, the advantage of magnetic resonance imaging (MRI) is the ability to provide combined haemodynamic and anatomical data, as well as tissue characterisation. A particular advantage of MRI is the absence of nephrotoxic contrast and radiation exposure. The choice of imaging modality depends on the specific structural heart intervention, patient-related factors, and institutional expertise. In some cases, echocardiography alone will suffice; however, in many cases the use of cardiac CT and/or MRI provides more comprehensive pre-procedural planning (Table 1). Below we summarise the role of each of these imaging modalities with specific structural heart interventional procedures.
Atrial and ventricular septal defects
Percutaneous technologies increasingly allow minimally invasive closure of clinically relevant atrial and ventricular septal defects. Closure of an atrial septal defect (ASD) is indicated in symptomatic or asymptomatic patients with right chamber enlargement and/or paradoxical embolism, while closure of a ventricular septal defect (VSD) is indicated with a haemodynamically significant shunt or history of endocarditis. Transthoracic echocardiography (TTE) and/or transoesophageal echocardiography (TEE) is the first-line diagnostic modality for assessment of both ASD and VSDs and their haemodynamic impact. Secundum ASDs with adequate surrounding rims are amenable to percutaneous closure, while the remainder typically require surgical repair (secundum ASDs without sufficient rim, and primum and sinus venosus ASD). In cases in which the defect cannot be clearly categorised, or in defects where the rims are not clearly visualised on TEE, CT with its high resolution and wider field of view can be useful. CT has been shown to have a particular advantage for assessment of large secundum defects with deficient inferior rims when compared to conventional TEE1. En face velocity-encoded cardiac MRI is also capable of providing similar incremental information, albeit at lower spatial resolution2.
The haemodynamic significance of ASDs and VSDs when uncertain by echocardiography can be reliably assessed using advanced non-invasive imaging tools. Although both CT and MRI allow accurate quantification of right chamber volumes and function, MRI has the added benefit of providing direct flow quantification across the ascending aorta and main pulmonary artery (Qp/Qs) without nephrotoxic contrast or radiation exposure.
Fistulas: coronary artery fistulas and patent ductus arteriosus
Congenital fistulas can pose problems in paediatric and adult patients. Coronary fistulas bypass myocardial capillaries and typically terminate in the ventricle (coronary-cameral fistula) or the pulmonary arterial tree (coronary arteriovenous fistula). Patients can develop a variety of symptoms including low-output heart failure, chest pain, pulmonary hypertension, arrhythmias, and syncope. CT or MRI can be invaluable in the diagnosis of coronary fistula. In fact, CT has been suggested to identify coronary fistulas better than invasive coronary angiography3. Compared to MRI, CT has better spatial and temporal resolution for coronary arterial assessment (particularly branch and distal vessels), and acquisition times are shorter. MRI can be an alternative choice in the evaluation of coronary fistulas, particularly for proximal coronary arteries using free-breathing sequences.
The ductus arteriosus connecting the main pulmonary artery to the aorta normally closes at birth. In rare instances of patency (PDA), the degree of left-to-right shunting determines the haemodynamic consequences that can include heart failure and pulmonary hypertension. While echocardiography is the first-line diagnostic tool, CT and MRI can also be useful, especially if poor acoustic windows limit echocardiography. CT can aid in characterising the size and degree of calcification surrounding the PDA, while MRI can be used to assess the haemodynamic effect of PDA by directly evaluating flow across the pulmonary artery and ascending aorta. Both modalities can help in selecting the appropriate therapy for PDA closure (either percutaneous options with coiling or occluder devices or surgical correction).
Alcohol septal ablation for hypertrophic cardiomyopathy
Both CT and MRI provide high spatial resolution for delineation of segmental myocardial thickness and identification of systolic anterior motion of the mitral leaflet. In addition, both modalities can provide detailed anatomical definition of the leaflets (including length of the anterior leaflet), papillary muscle, and chordal anatomy. Accurate assessment of segmental myocardial thickness is particularly important, as it was recently demonstrated that septal ablation is unlikely to be successful in patients with marked septal hypertrophy (either basal anteroseptal >24 mm or total thickness of basal anteroseptal and basal anterior segment >51 mm)4. CMR provides high-resolution, accurate measurements of myocardial thickness, and is more accurate than echocardiography. CMR is currently performed routinely in patients with hypertrophic cardiomyopathy for confirmation of diagnosis, exclusion of other aetiologies of hypertrophy such as Fabry disease or amyloid heart disease, assessment of severity of outflow tract obstruction (although echocardiography is typically adequate for this purpose), and prognostic assessment based upon the extent and severity of abnormal late gadolinium enhancement.
CT can also provide highly accurate segmental myocardial thickness measurements. In addition, the advantages of coronary CT angiography include the simultaneous exclusion of epicardial coronary stenosis, and identification of potential septal perforator branches for planning of alcohol septal ablation procedures. This is of importance as a recent study reported that, of patients undergoing alcohol septal ablation, 15% had ablation of a septal perforator artery culprit that did not originate from the left anterior descending artery, of whom half had prior unsuccessful septal ablation procedures5.
Pulmonary vein stenosis stenting
Both CT and MRI have been used for pulmonary vein mapping prior to ablation procedures for atrial fibrillation. The use of one imaging modality over another is related to institutional factors, though MRI is an attractive option in patients with renal insufficiency. Modern-generation CT scanners which facilitate high-pitch prospective helical acquisition modes allow scans to be acquired with an effective dose of about 1 mSv6. For assessment of pulmonary vein stenosis, it may be preferable to utilise the modality used for pre-ablation planning for direct comparison. MRI has the advantage of direct interrogation of the velocity and flow at each of the pulmonary veins, in cases in which echocardiography is not adequate.
Paravalvular regurgitation
Paravalvular regurgitation (PVR) from surgically placed prosthetic heart valves and more recently transcatheter valve replacement (most commonly in the aortic position) is a challenging complication that is increasingly treated with percutaneously placed occluder devices. These complications are frequently identified via echocardiography; however, CT and MRI are often necessary for better assessment of the regurgitant orifice, especially when the acoustic windows are suboptimal for echocardiography due to reverberation and shielding from the prosthetic valve7-9. Both modalities (in particular CT) allow detailed sizing of the orifice, can aid in selection of occluder devices, and can be utilised for planning angiographic angles utilised during percutaneous closure (Figure 1). Additionally, both techniques allow simultaneous interrogation of important adjacent anatomic structures (in the case of aortic valve PVR, the left ventricular outflow tract and coronary ostia). Moreover, MRI permits quantitative assessment of the degree of PVR unlike the more subjective and semi-quantitative methods frequently used in echocardiography. Furthermore, MRI evaluation of PVR has been shown to carry important prognostic information. In a recent study, MRI provided superior prognostic value to echocardiography after TAVR, as those patients with greater than mild paravalvular regurgitation by MRI had a higher incidence of adverse events10.
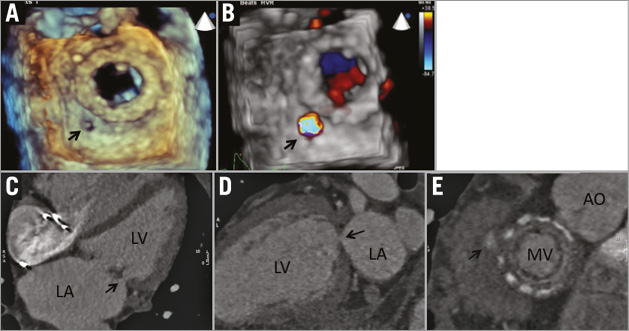
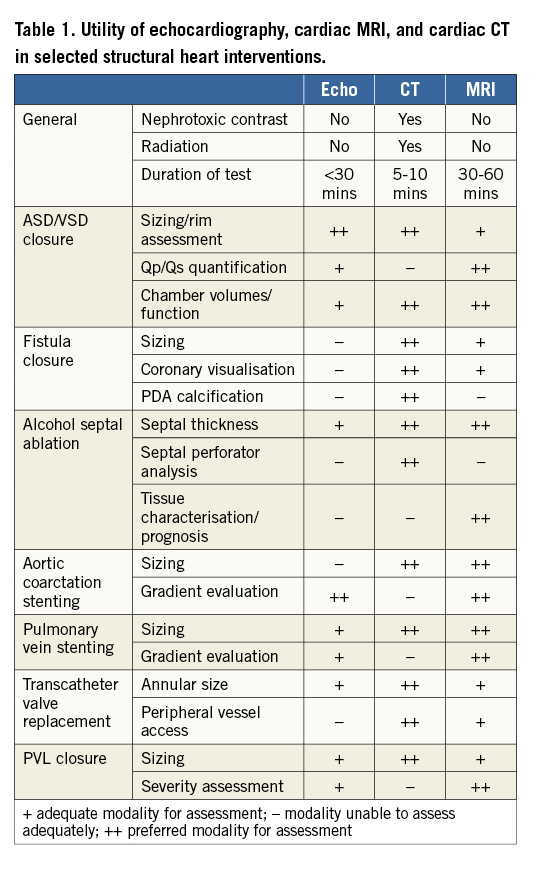
Figure 1. Mitral paravalvular leak located posterolaterally (8 o’clock) on en face view from left atrial perspective. A) & B) 3D-TEE shows location with colour Doppler flow. C) & D) CT images showing the defect in orthogonal views (arrows), and CT short-axis reconstructed image of the mitral valve prosthesis with posterolateral defect. CT allowed accurate sizing of the defect (4×3 mm). This symptomatic heart failure patient underwent successful closure with a 6 mm AVP II closure device with complete reduction of mitral regurgitation from 2-3+ to 0. E) Analogous view on CT to 3D-TEE en face view of mitral valve. Note posterolateral defect (arrow).
Coarctation of the aorta
Children with symptomatic coarctation of the aorta (CoA) typically undergo surgical resection at a young age. Residual stenosis is common and in some instances post-stenotic aneurysm formation occurs. Monitoring with echocardiography is challenging as the imaging windows often become poor as the child grows. Repeated examination with CT leads to cumulative radiation exposure while multiple cardiac catheterisations are invasive and also expose the patient to radiation. As such, MRI is often utilised (Figure 2). Haemodynamic data from MRI have been shown to correlate well with pressure gradients across the coarctation measured by invasive catheterisation.
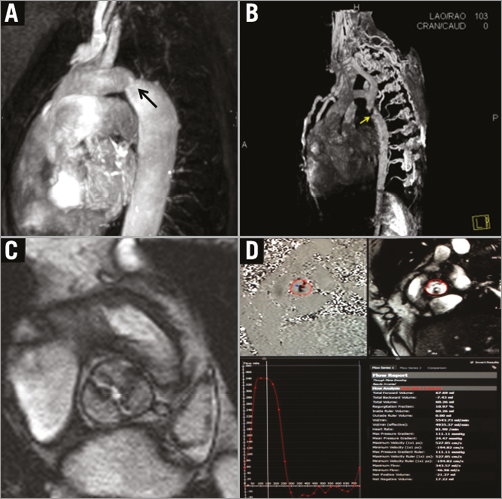
Figure 2. Evaluation of aortic coarctation with contrast-enhanced MRI. Patient with mild-moderate post-ductal coarctation (A) and marked coarctation/interruption of the descending thoracic aorta with extensive posterior intercostal collaterals (B). MRI can simultaneously evaluate aortic valve morphology (note bicuspid aortic valve morphology in C) and severity of stenosis by phase contrast imaging (D). Phase-contrast MRI can be utilised for quantitative assessment of gradients across the coarctation.
Transcatheter aortic and mitral valve replacement (TAVR/TMVR)
Since CT has superior spatial resolution and allows multiplanar imaging of the entire aorta and peripheral vasculature, it has emerged as the preferred pre-TAVR imaging modality for determination of annular size, coronary ostial distance from the annulus, and comprehensive assessment of peripheral vessels to guide route of access (Figure 3). However, in cases of renal impairment or arrhythmia, MRI is an attractive alternative to CT that does not require contrast administration and is less dependent on heart rate. In a study of 50 patients referred for TAVR who underwent multimodality pre-TAVR imaging with both MRI and CT, no differences were seen between MRI and MDCT in calculation of aortic annulus area11. In addition, there was excellent correlation between MRI and MDCT in the determination of aortic annulus root geometry and coronary ostial height. A notable shortcoming of MRI is its relative inability to evaluate the burden of calcification which is important to identify the best percutaneous access approach. An emerging application of CT is its utility in the planning of transcatheter mitral valve interventions. Similar to TAVR, dedicated CT software packages have emerged allowing mitral annular measurements and procedural planning. Given the greater complexity of mitral valve anatomy, CT has a very important role in the pre-procedural planning of TMVR. Similar to TAVR assessment, its utility in TMVR is multifold, including assessment of valve and adjacent anatomical structures, determination of accurate annulus dimensions for prosthesis sizing, vascular access planning, and prediction of fluoroscopic angles (Figure 4).
Figure 3. Computed tomography planning for TAVR. Post-processing software can be utilised to identify the virtual annular ring formed by the nadir of each of the attachments of the aortic cusps (cvi42®; Circle Cardiovascular Imaging, Calgary, AB, Canada) (A), and to evaluate peripheral vascular anatomy suitability for transfemoral artery access (B & C).
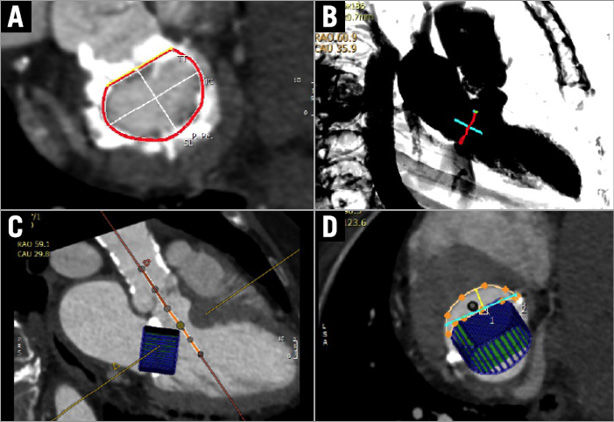
Figure 4. Computed tomography planning for TMVR. Post-processing software can be utilised to segment the mitral annulus for device sizing (A), plan transapical access (B), and simulate specific device sizes to predict the likelihood of left ventricular outflow tract obstruction (C & D).
Left atrial appendage closure
Anticoagulation is necessary to prevent cardioembolic stroke in at-risk patients with atrial fibrillation. However, despite the development of direct oral anticoagulants, the risk for bleeding over a lifetime of anticoagulation is not trivial. As such, there has been growing interest in percutaneous solutions for left atrial appendage (LAA) closure. While TEE is typically used to assess the LAA, the procedure is invasive, requires sedation, and is occasionally limited by poor windows. The LAA is a complex anatomical structure with a significant patient-to-patient variability in size, shape, and precise location. CT has emerged as a vital pre-procedural tool to visualise the anatomy of the LAA and adjacent structures. There are a number of LAA closure devices available to the clinician and CT aids in device selection. For instance, the LARIAT® device (SentreHEART, Inc., Redwood City, CA, USA) is contraindicated in an LAA that courses posterior to the pulmonary artery. Furthermore, CT can evaluate patients post procedure for incomplete occlusion or procedural complications. The presence of contrast (or blood flow) in the LAA post occlusion suggests incomplete closure12. In cases of incomplete LAA closure, progressive endothelialisation of the gap between the LAA ridge and device may be monitored serially with CT and potentially help determine when to discontinue anticoagulation. While periodic monitoring with TEE is commonplace, imaging with CT provides a non-invasive alternative.
Clearly, optimal pre-procedural planning with imaging is associated with greater procedural success. Cardiac CT and MRI are complementary tools to conventional echocardiography and invasive catheterisation which are likely to have an increasing role in planning structural heart interventions.
Conflict of interest statement
The authors have no conflicts of interest to declare.

