Abstract
Aims: Early data on the Edwards SAPIEN 3 valve (S3-THV) have shown low rates of paravalvular leaks and vascular complications but relatively high 30-day permanent pacemaker implantation (PPMI) rates. No direct comparisons on clinical outcomes including PPMI rates are available for the S3-THV and the Edwards SAPIEN XT (XT-THV). We aimed to compare the 30-day PPMI rates in patients treated with the two prostheses and to assess the interplay among valve type, depth of implantation and PPMI rate.
Methods and results: Two hundred and nine patients treated by TAVI were considered. The S3-THV was associated with higher PPMI rates compared to the XT-THV, both overall and in subgroups matched for several predictors of PPMI. However, in the S3-THV group, 30-day PPMI was strictly associated with deep valve implantation, and PPMI risk of high-implanted S3-THVs was similar to that of the overall XT-THV matched group. No cases of significant paravalvular leak were observed in the S3-THV group.
Conclusions: The S3-THV was associated with a higher incidence of PPMI compared to the XT-THV. In the S3-THV group, pacemaker implantation was strictly associated with deep valve implantation. An implantation technique involving higher initial placement of the central marker (from 0 to 3 mm above the base of the aortic cusps) and, as a consequence, higher final valve depth might help in preventing post-TAVI PPMI with the S3-THV, without affecting the risk of paravalvular leak.
Abbreviations
PPMI: permanent pacemaker implantation
S3-THV: Edwards SAPIEN 3 transcatheter heart valve
STS: Society of Thoracic Surgeons
VARC-2: Valve Academic Research Consortium-2
XT-THV: Edwards SAPIEN XT transcatheter heart valve
Introduction
Transcatheter aortic valve implantation (TAVI) is an established alternative for patients with aortic valve stenosis and at high risk for surgery or who are inoperable1,2. The occurrence of atrioventricular (AV) conduction disturbances is common after TAVI and may lead to early permanent pacemaker implantation (PPMI) with rates ranging widely in the literature, from 2% up to 51%3. A number of patient-related and procedure-related factors, including valve type, have been recognised as being related to the risk of AV conduction defects requiring PPMI after TAVI4-9. In particular, multiple studies have outlined that the use of balloon-expandable valves may significantly lower the risk for PPMI as compared to self-expandable ones3,9. Among balloon-expandable valves, the Edwards SAPIEN family of transcatheter heart valves (Edwards Lifesciences, Irvine, CA, USA) has shown very favourable outcomes so far, in particular for the second-generation Edwards SAPIEN XT (XT-THV), with an overall reported rate of post-TAVI PPMI of approximately 6%3. Compared to the XT-THV, the recently introduced Edwards SAPIEN 3 valve (S3-THV) incorporates a new metallic frame to allow a low delivery profile while keeping a radial strength similar to the XT-THV, and an external fabric cuff to minimise paravalvular leaks. The S3-THV valve frame has a higher profile compared to the XT-THV and, if a similar implantation technique is adopted, it may extend deeper into the left ventricle outflow tract after deployment10,11. In this regard, preliminary data on the S3-THV device from the pivotal SAPIEN 3 trial have shown a very low rate of paravalvular leaks and vascular complications but an increased 30-day PPMI rate (13.3%). No head-to-head comparisons on clinical outcomes (including PPMI rates) are currently available for the S3-THV and the XT-THV. Thus, we aimed to compare 30-day PPMI rates in patients treated with the two prostheses. Furthermore, we aimed to assess the interplay among valve type, depth of implantation and PPMI rate.
Methods
All patients treated by TAVI for aortic stenosis at our institution with the XT-THV and S3-THV between July 2010 and July 2014 were considered for inclusion. For the purposes of this analysis, patients with previous pacemaker implantation were excluded. Patients were assessed by clinical evaluation, 12-lead ECG, fluoroscopy and echocardiography. All patients underwent clinical, ECG and echocardiographic follow-up pre-discharge and at 30 days. Data were collected in a dedicated database. This study was performed in accordance with the Declaration of Helsinki, all patients gave written consent for treatment and data collection, and the study was approved by the local ethics committee.
Risk factors and endpoints
Preoperative risk-related variables were defined according to the EuroSCORE definitions and outcomes were reported according to VARC-2 definitions12. The patients were classified as receiving an S3-THV or an XT-THV valve. Our primary study endpoint was the 30-day rate of PPMI with class I or class IIa indication according to current guidelines. In order to avoid bias related to a different threshold for pacemaker implantation in the treatment arms, “preventative” pacemaker implantation (defined as a class IIb indication for PPMI) was not included in the primary study endpoint. Since dedicated guidelines for PPMI in the setting of TAVI are currently lacking, the class of indication for PPMI was defined according to guidelines for the general population13,14. Secondary endpoints were the occurrence within 30 days post procedure of all-cause mortality, cardiac death, stroke, myocardial infarction, cumulative major adverse cardiac events (MACE), cumulative early safety and clinical efficacy (definitions in Online Table 1), procedural success, congestive heart failure requiring hospital re-admission, intravalvular and paravalvular aortic regurgitation, and prosthetic valve dysfunction.
Fluoroscopy
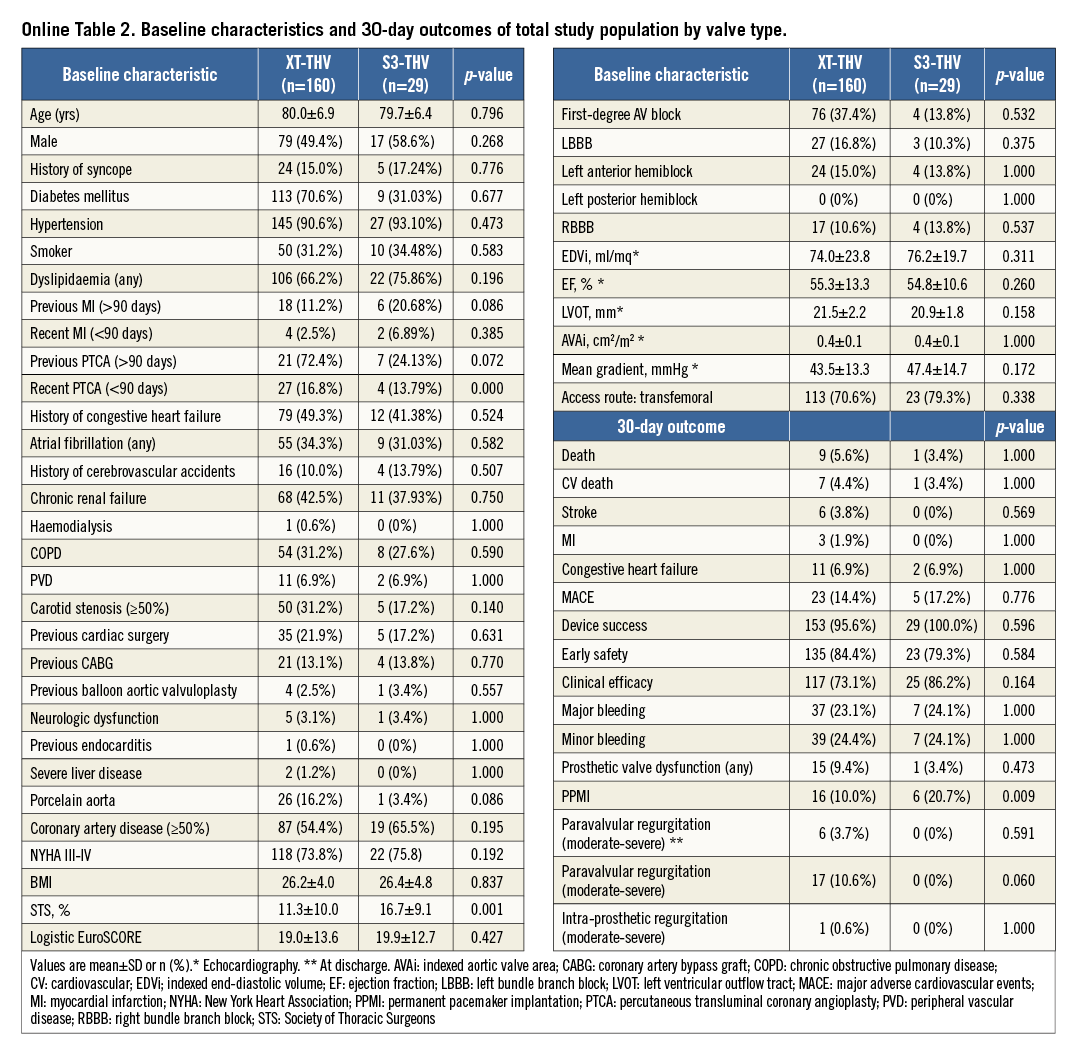
In the present study, valve implantation depth was assessed based on off-line evaluation of procedural fluoroscopy. A single still frame of the post-deployment aortic angiogram was considered for each patient. In all patients, final aortic angiograms were performed after adjustment of the projection angle in order to obtain an orthogonal view of the valve frame post deployment, thus avoiding inaccuracy in depth measurements. Valve implantation depth was assessed by two different methods. A qualitative (visual) assessment was performed separately by two experienced implanters (MN and GI), who estimated the aorto-ventricular ratio of the valve frame position. Inter-observer discrepancies were resolved by consensus. The position of the valve frame was also categorised as high when the aorto-ventricular ratio was higher than 60/40 and as low when the aorto-ventricular ratio was equal to or lower than 60/40. Furthermore, a quantitative assessment was performed by measuring the maximum distance of valve frame inflow from the native aortic annulus, and the distance on the septal and non-septal sides. The native aortic annulus was identified by tracing a line intersecting the hinge point between the profile of the valve frame and the neighbouring sinus of Valsalva (Figure 1). Angiograms were evaluated prior to all statistical analyses, and fluoroscopy readers were blinded in relation to outcome measures. Quantitative measurements were performed with the OsiriX software, version 5.0.2.
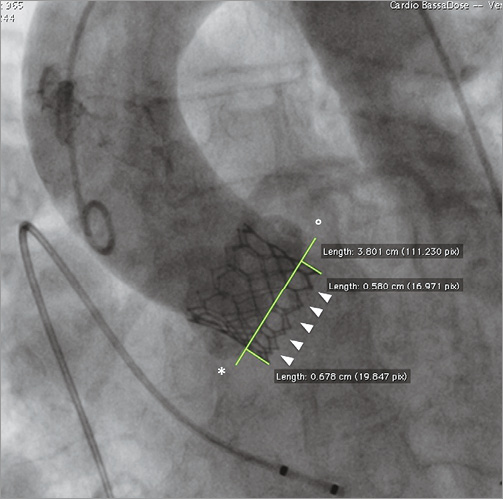
Figure 1. Quantitative assessment of valve implantation depth of an Edwards SAPIEN 3 valve. Optimal alignment of valve inflow (white arrowheads) has been obtained with post-deployment adjustment of the angiography projection angle. Valve depth is measured both on septal (*) and non-septal (°) sides of the valve frame with respect to aortic annulus assessed as being in correspondence with a line intersecting the hinge point between the profile of the valve frame and the neighbouring sinus of Valsalva.
Statistical analysis
Quantitative variables were expressed as mean±standard deviation and compared between valve types with the Student’s t-test in case of normality or the Wilcoxon rank-sum test. Categorical variables were expressed as counts and percentages and compared between valve types using the χ2 test or Fisher’s exact test, as appropriate. Since the selection of valve types was not randomised in this observational study, we applied a propensity score matched method to control selection bias based on an extensive number of possible or well recognised predictors for post-TAVI AV conduction defects3-9. The propensity score was estimated with a logistic regression model including the following variables to predict the use of a specific valve type: age, gender, history of atrial fibrillation, baseline conduction disturbances (including first-degree AV block, second-degree AV block, left bundle branch block, right bundle branch block, left anterior hemiblock, left posterior hemiblock, left axis deviation), QRS duration, left ventricular ejection fraction, left ventricular outflow tract diameter, baseline aortic regurgitation grade, access route, balloon pre- and post-dilatation, prosthesis size, percentage valve oversizing, STS mortality risk, logistic EuroSCORE. Valve implantation depth was excluded, being a covariate for subsequent logistic regression analysis. A greedy, nearest neighbour 1:1 matching algorithm was used to match the two groups of patients on the logit of the propensity score (using callipers of width equal to 0.2 times the standard deviation of the logit of the propensity score). Standardised differences were used to compare patients’ demographic and clinical characteristics and a d-value ≤0.2 was considered indicative of a good balance. The comparison of outcome variables between valve groups after matching was conducted with the McNemar’s test. In the S3-THV group, a ROC curve was obtained on all possible valve depth cut-offs according to the occurrence of PPMI. Subsequently, logistic regression was performed to assess the relationship between valve type and implantation depth. A scatter graph was obtained for the S3-THV group to assess the relationship between final valve implantation depth and the pre-deployment position of the central marker. All reported p-values are two-sided, and p-values of less than 0.05 were considered statistically significant. Statistical analyses were performed with SAS version 9.1.3 for Windows (SAS Institute Inc., Cary, NC, USA). Data used for this analysis were extracted on July 25, 2014.
Results
A total of 209 patients treated by TAVI (179 with the XT-THV and 30 with the S3-THV) were considered. Due to pacemaker implantation before TAVI procedure, 19 patients in the XT-THV group and one patient in the S3-THV group were excluded. Baseline characteristics and 30-day outcomes of the total study groups are reported in Online Table 2. All baseline characteristics except STS mortality risk and indexed aortic valve area were comparable in the total populations. Outcomes were similar with the exception of the study primary endpoint (30-day PPMI rate 10% in the XT-THV group and 20.7% in the S3-THV group, p=0.009). In the total population, pacemaker implantations included in the primary endpoint were performed with the following class I-IIa indications: third-degree AV block (persistent 85.4%, paroxysmal 9.9%) and persistent bradycardia due to second-degree type 2 AV block (4.8%). “Preventative” PPMI (excluded from primary endpoint calculation) occurred in one patient (3.4%) in the S3-THV group and in five patients (3.1%) in the XT-THV group.
The rate of moderate-severe paravalvular regurgitation at 30 days was lower in the S3-THV group compared to the XT-THV group (0% versus 10%, p=0.060). After matching, 29 patients in each valve group were available for analyses. The baseline characteristics and 30-day outcomes of the matched subgroups are shown in Table 1. The PPMI rate was lower in the XT-THV group compared to the S3-THV group (3.44% versus 20.68%, p<0.001, respectively). All other outcomes were comparable between groups. The depth of valve implantation was significantly lower in the S3-THV group compared to the XT-THV group, both at quantitative (maximum depth, 5.2±2.3 mm versus 8.6±3.9 mm, p<0.001, respectively) and at qualitative assessment (aorto-ventricular ratio ≤60/40, 52.9% versus 34.5%, p=0.046). Specifically, in the S3-THV population, the depth of valve implantation (both quantitative and qualitative) was significantly lower in patients who required PPMI within 30 days versus patients without the need for PPMI (maximum depth, 8.2±2.0 mm vs. 5.0±2.4 mm, p=0.032; aorto-ventricular ratio ≤60/40, 76.0% versus 47.6%, p=0.036). By ROC curve analysis, a valve depth less than 8 mm resulted as the best compromise in terms of reduction of the PPMI rates for the S3-THV (sensitivity 63%, specificity 86%, area under the curve, 71.4%). Figure 2 shows the relationship among valve type, depth of implantation and PPMI rates. With respect to the relationship between the pre-deployment position of the central marker and valve implantation depth, when the initial position of the central marker was from 0 to 3 mm above the “base-of-the-cusps” line, the final S3-THV depth was never ≥8 mm (Figure 3).
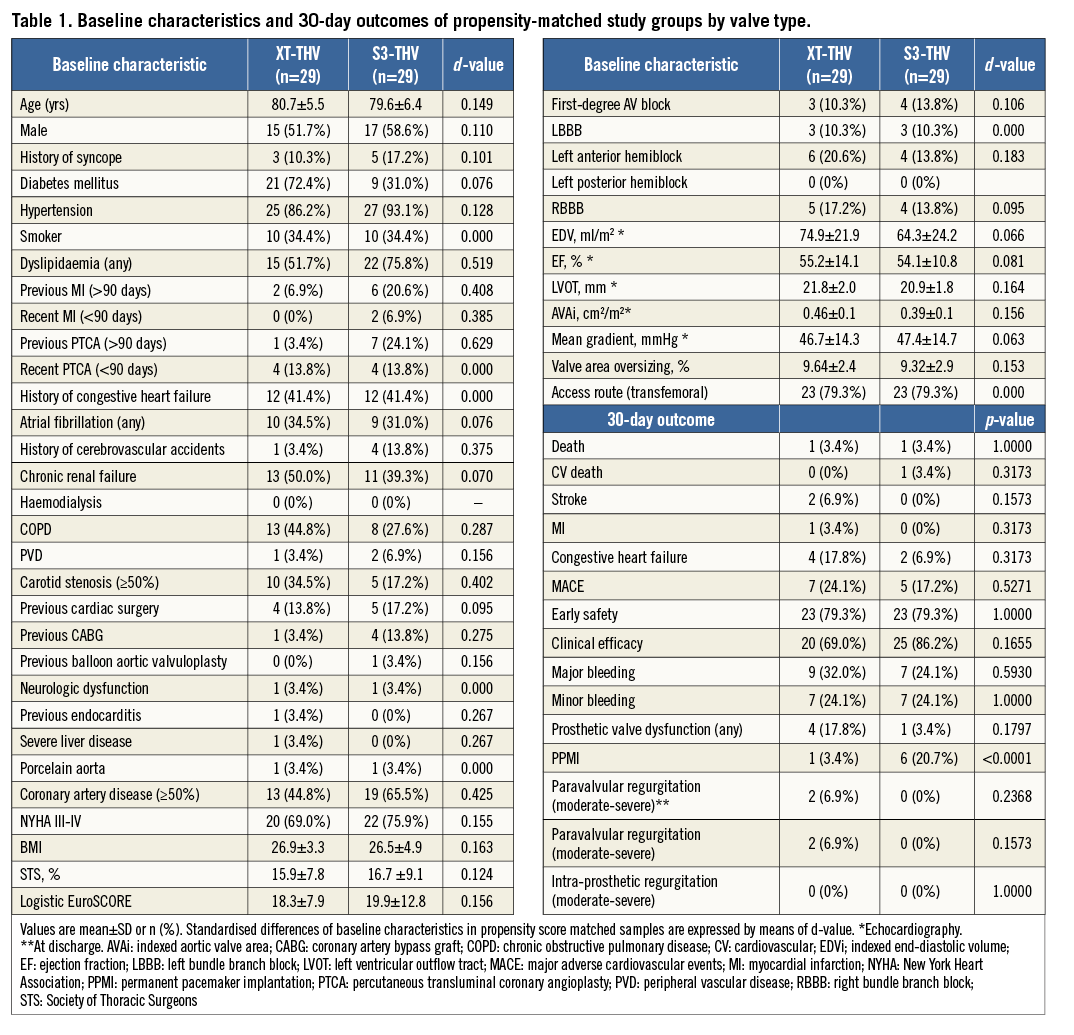
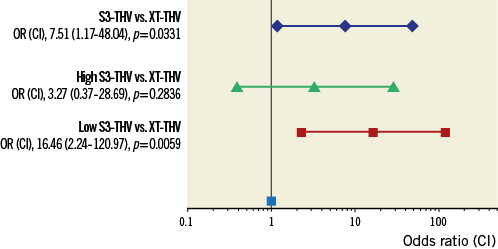
Figure 2. Risk of PPMI by valve type expressed as odds ratio (confidence interval). In matched groups, the risk of PPMI was significantly higher for the S3-THV compared to the XT-THV. However, this higher risk for PPMI was driven by S3-THV patients who received a low implantation (valve implantation depth ≥8 mm). When comparing the XT-THV group with high-implanted S3-THV patients only, a similar risk for PPMI was observed.
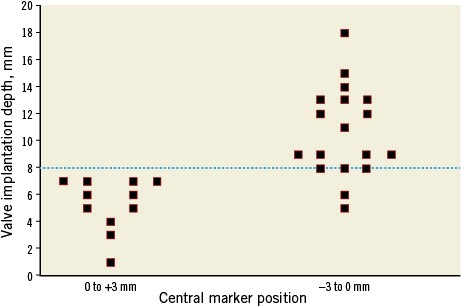
Figure 3. Scatter plot of final valve depths in the S3-THV group (quantitatively assessed) by the initial position of the central marker before initial valve deployment. In all cases valve implantation resulted in a high final frame position (depth less than 8 mm) when the initial position of the central marker was above the so-called “base-of-the cusps” line.
Discussion
The main findings of the present study are the following: 1) the S3-THV was associated with higher PPMI rates in comparison to the XT-THV both in the overall population and in matched subgroups; 2) in the S3-THV group, the occurrence of 30-day PPMI was associated with lower valve implantation, either quantitatively or qualitatively assessed; 3) the risk of PPMI was not statistically different between high-implanted S3-THVs and the overall XT-THV matched group; and 4) no cases of moderate-severe paravalvular leak at 30 days occurred in the S3-THV group.
The need for permanent pacemaker implantation and the occurrence of paravalvular leaks are two major drawbacks of TAVI because of the correlation of both these complications with prognosis, even if inconsistency is present in the literature regarding the impact of PPMI on subsequent outcomes15-19. One of the main purposes of the recently CE-mark approved S3-THV is to address the major issue of paravalvular leaks by means of a dedicated sealing system located externally in the inferior part of the valve frame. Preliminary data at 30 days from the pivotal SAPIEN 3 trial have confirmed a very low incidence of moderate-severe paravalvular regurgitation with the S3-THV (3.4%) but an increased rate of PPMI (13.3%). Accordingly, in our study we confirmed the reduction in PVL rate but also the increase in the occurrence of PPMI at 30 days after implantation of the S3-THV compared to the XT-THV. However, higher valve implantation (ventricular ratio >60/40 at qualitative assessment or depth <8 mm at quantitative assessment) significantly reduces the risk of PPMI with the S3-THV, without affecting the risk of paravalvular leak development or valve malposition. These data are consistent both with the early experiences with the XT-THV as well as with pathology findings showing that the AV conduction system arises on the left side of the interventricular septum at least 7 mm below the inter-leaflet triangle located between the right and the non-coronary cusps20-22. In view of this, a valve depth <8 mm as a “safety threshold” might prevent direct interactions between the valve frame and the AV conduction system. Current practice for S3-THV implantation is based on the placement of the central marker across the native valve within a few millimetres above or below the “base-of-the-cusps” line before valve deployment. To be pragmatic, based on our results, we propose that the initial central marker of the crimped S3-THV should be located within 3 mm above the “base-of-the-cusps” line in order to minimise the need for PPMI (Figure 4). In this context, it should also be recognised that, in case of high implantation, the new design of the S3-THV (in particular the modification of the stent frame in its upper segment) may allow safer coverage of the left main stem, something which XT-THV users generally tried to avoid (Figure 4).
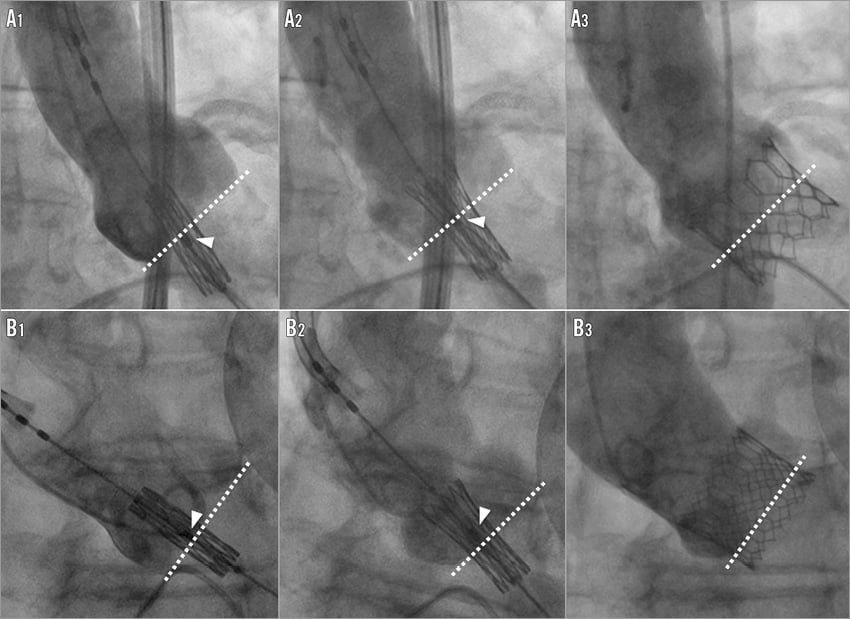
Figure 4. Two opposite clinical scenarios of low (A1-A3) and high-implanted (B1-B3) Edwards SAPIEN 3 THVs, illustrating the relationship between the initial position of the central marker with respect to the “base-of-the cusps” line and the final valve implantation depth. A1) Before starting valve deployment, the central marker (arrowhead) is placed below the “base-of-the-cusps” line. A2) During the initial deployment, the central marker slightly shifts upwards towards the base of the cusps. A3) Implantation results in a low final valve frame position (maximum depth 9.2 mm, aorto-ventricular ratio 50/50). B1) The initial position of the central marker (arrowhead) is above the base of the cusps. B2) The central marker remains in a stable position during mild balloon inflation. B3) After complete deployment, the S3-THV is located in high position (maximum depth 4.1 mm, aorto-ventricular ratio 80/20).
Limitations
The following limitations of this study must be considered: 1) this is a small, non-randomised, observational study from a single, high-volume centre; 2) even if propensity matching is a well-established method to reduce the impact of treatment selection bias using observational data and to replicate some of the features of a randomised controlled trial, the characteristics of propensity-matched populations may still not be perfectly identical; 3) the two valve types have been implanted simultaneously at our institution only for a limited time period; 4) fluoroscopy imaging and echocardiography imaging were not interpreted at an independent core lab; 5) in this study, calcium distribution as a potential factor influencing the risk of conduction disturbances was not systematically investigated, and thus calcium distribution assessment was not included in the analyses, even if it needs to be recognised that the impact of calcium distribution in the LVOT on conduction disturbances is currently uncertain and standardised methods for its assessment in this context are not unequivocally established; 6) CT scan is the gold standard technique for valve depth assessment. However, postoperative, research-oriented CT scan might be complex to obtain in consecutive patients due to ethical and logistic issues. To address this limitation, valve implantation depth was assessed on post-deployment aortic root angiograms optimised for valve frame orientation (as detailed in the “Methods” section).
Conclusions
In this study, the S3-THV prevented the occurrence of moderate-severe periprosthetic aortic regurgitation in all patients at 30 days post procedure, regardless of valve implantation depth. On the other hand, the S3-THV was associated with a higher incidence of major AV conduction defects requiring PPMI compared to the XT-THV, both in total populations and in subgroups matched for several predictors of PPMI. However, in the S3-THV group, PPMI was associated with deeper valve implantation. Furthermore, the risk of PPMI was higher for the low-implanted S3-THV group compared to the XT-THV group, while it was similar in the high-implanted S3-THV group compared to the XT-THV group. In the light of these results, a high S3-THV implantation is preferable and might help in preventing post-TAVI AV conduction defects requiring PPMI. From a clinical standpoint, a slight modification of the implantation technique of the Edwards SAPIEN 3 to get a higher final aorto-ventricular ratio may improve significantly the outlook of patients treated with this kind of valve in terms of the need for permanent pacemaker implantation.
| Impact on daily practice The Edwards SAPIEN 3 transcatheter heart valve is expected shortly to replace the Edwards SAPIEN XT valve in clinical practice, but no direct comparisons on major clinical endpoints including PM rates are currently available for these two devices. Clinicians who are involved in patient care in case of TAVI should be aware of an increased risk of AV conduction defects with the SAPIEN 3 valve compared to the SAPIEN XT. However, a minor modification of the implantation technique of the Edwards SAPIEN 3, involving a higher final valve depth, may avoid AV conduction defects after TAVI in a significant number of patients, without affecting the risk of paravalvular leak development. |
Conflict of interest statement
G. Tarantini, G. Isabella, and G. Gerosa have received lecture fees from Edwards Lifesciences. The other authors have no conflicts of interest to declare.
Online data supplement