
Abstract
Aims: The aim of the study was to assess the outcomes of balloon aortic valvuloplasty (BAV) as a rescue therapy in patients with cardiogenic shock (CS) related to severe aortic stenosis (AS).
Methods and results: Forty-four consecutive patients, n=31 with hypotensive CS (HCS) and n=13 with non-hypotensive CS (NHCS) due to acutely decompensated severe AS, from two centres were treated with urgent BAV. The composite primary endpoint was mortality or recurrent CS at one-year follow-up. These patients (77.3±8.1 years old; 75% male) had a mean EuroSCORE II of 41.6±13.7%. One-month mortality was 47%. Twelve patients (27%) had either a staged TAVR (n=10) or surgical aortic valve replacement (SAVR) (n=2) with a median delay of 79 days after BAV: n=6 (19%) in the HCS subgroup and n=6 (46%) in the NHCS population (p=0.06). At one year, the rate of composite all-cause death or recurrent CS was 75% and significantly higher in the HCS subgroup (83% vs. 53%; p=0.03). Overall one-year mortality was 70% (n=31) with a trend for a better prognosis in NHCS patients (54% vs. 77%; p=0.09). Univariate predictive factors of the primary endpoint included preoperative dose of dobutamine >5 microg/kg/min (100% vs. 57%; p=0.001) and delayed BAV >48 hrs (90% vs. 59%; p=0.01).
Conclusions: Despite the initial success of urgent BAV, morbidity and mortality of CS related to severe AS remain high and directly related to the time of the valvuloplasty. Performing BAV before or within 48 hours of starting inotropic agents appears to be key to survival.
Abbreviations
AS: aortic stenosis
AVR: aortic valve replacement
BAV: balloon aortic valvuloplasty
BMI: body mass index
CS: cardiogenic shock
GFR: glomerular filtration rate
HCS: hypotensive cardiogenic shock
LVEF: left ventricular ejection fraction
NHCS: non-hypotensive cardiogenic shock
PASP: pulmonary arterial systolic pressure
SAVR: surgical aortic valve replacement
TAPSE: tricuspid annular plane systolic excursion
TAVR: transcatheter aortic valve replacement
TTE: transthoracic echocardiography
VARC-2: Valve Academic Research Consortium-2
Introduction
Management of patients with cardiogenic shock (CS) related to severe aortic stenosis (AS) is a challenging topic1. These patients have a poor prognosis with high morbidity and mortality (50-75%)2,3 and a high operative risk for surgical aortic valve replacement (SAVR) (up to ≈25% operative mortality)4. Percutaneous balloon aortic valvuloplasty (BAV) is a therapeutic option described by Cribier et al in 19865, particularly for the treatment of patients with CS related to severe AS6. After initial enthusiasm in the 1990s, the use of BAV decreased dramatically, because of the early high restenosis rate (70%), and it was reserved for palliative indications7.
For a decade, transcatheter aortic valve replacement (TAVR) has been an alternative to medical treatment for those who are contraindicated to surgery8,9. Recently, there has been renewed interest in BAV10,11 because, as stated in the ESC guidelines for the management of valvular heart disease, “BAV may be considered as bridge to surgery or TAVR in haemodynamically unstable patients who are at high risk for surgery”12.
However, only a few single-centre studies including small cohorts of patients with CS (n=7 to 23 patients) have been conducted10,11,13-16, most of them before the TAVR era13,14. More recently, a multicentre study investigated the role of BAV in emergency conditions but did not clarify the role of BAV in patients with cardiogenic shock17.
The purpose of the present study was to assess the periprocedural and one-year outcomes of BAV as a rescue therapy in contemporary patients with CS due to severe AS.
Methods
PATIENT SELECTION
Between 2011 and 2016, we identified consecutive patients suffering from CS related to severe AS referred to the intensive care units of two French university centres (APHM Marseille and CHRU Lille). Each case was discussed by the institutional multidisciplinary Heart Team, which included on-call interventional cardiologists and surgeons, and on-site intensive care physicians and anaesthetists. The Heart Team agreed that a BAV should be performed as life-sustaining rescue therapy for every patient, as emergency aortic valve surgery was excluded.
In those patients, cardiogenic shock was defined as the combination of 1) a low cardiac index less than 2.2 L/min/m2 (transthoracic echocardiography [TTE] evaluation) together with clinical signs of pulmonary congestion resistant to a high dose of intravenous loop diuretic treatment, and 2) peripheral hypoperfusion identified by the combination of several parameters including altered mental status, cold/clammy skin and extremities, oliguria with urine output of less than 30 ml/hr, or serum lactate level higher than 2.0 mmol/L.
As proposed by Menon et al from the SHOCK trial registry18, two “subsets” of patients with CS were defined, non-hypotensive CS (NHCS) and hypotensive CS (HCS), as they are continuous pathophysiological conditions.
(i) Non-hypotensive or normotensive-hypoperfused CS is defined as above by the combination of low cardiac output/peripheral hypoperfusion together with a “normal” systolic blood pressure >90 mmHg without vasopressor circulatory support.
(ii) “Classic” CS or hypotensive CS (HCS), as in the IABP-SHOCK II trial19, is defined as above by the combination of low cardiac output/peripheral hypoperfusion together with a low systolic blood pressure of less than 90 mmHg for more than thirty minutes or infusion of inotrope drugs needed to maintain a systolic pressure above 90 mmHg.
All patients had severe AS (indexed aortic valve area [AVA] <0.6 cm2/m2).
Patients with CS related to other causes such as ST-segment elevation myocardial infarction (STEMI), tamponade, stress cardiomyopathy, pulmonary embolism, myocarditis, severe aortic regurgitation, severe mitral regurgitation/stenosis, or patients with concomitant sepsis or severe bleeding were excluded. No right cardiac catheterisation or PiCCO® (Maquet, Orleans, France) was performed, but an electrocardiogram, echocardiography, biological assessments and angiocoronarography were performed before the procedure to confirm the diagnosis.
PREPROCEDURAL SCREENING
Patients received standard care therapy as previously described20. Invasive blood pressures were monitored with arterial and venous catheters. Patients with hypotensive CS were all catecholamine (dobutamine and/or norepinephrine)-dependent at baseline.
Severe AS was assessed according to the guidelines of the European Society of Cardiology12.
BAV PROCEDURES
A coronarography angiogram was systematically performed before the BAV in order to exclude patients with significant left main or proximal left anterior disease.
BAV was performed using rapid pacing as previously described6 under local anaesthesia, through the transfemoral access. The procedure was considered successful when at least a 50% reduction of the aortic gradient was obtained without a moderate to severe aortic regurgitation6. This was assessed by TTE after the procedure.
CLINICAL ENDPOINTS
The primary endpoint was a composite of mortality or recurrent CS related to AS at one-year follow-up, and secondary endpoints included one-year mortality and predictive factors of the primary endpoint and one-year mortality. Other analyses included one-month mortality and post-procedural outcomes, and were described according to the Valve Academic Research Consortium-2 (VARC-2) criteria21.
STATISTICAL ANALYSIS
Results for continuous variables were expressed as means with standard deviations when data were symmetrically distributed or otherwise as medians with ranges. The normality of distribution was assessed using the Shapiro-Wilk test and normality diagrams. Results for categorical variables were expressed as frequencies and percentages. Comparative analyses were obtained using the chi-square test for categorical data; when not applicable because of the sample size, Fisher’s exact test was used. For numerical variables, we used the ANOVA test or Kruskal-Wallis test if normality of distribution was not present. Survival was graphically depicted using Kaplan-Meier curves and between-group differences were compared using the log-rank test. P-values <0.05 were considered statistically significant. Statistical analysis was performed using commercial software (SPSS version 18.0; SPSS Inc., Chicago, IL, USA).
A multivariate analysis was not possible because of the low number of patients.
Results
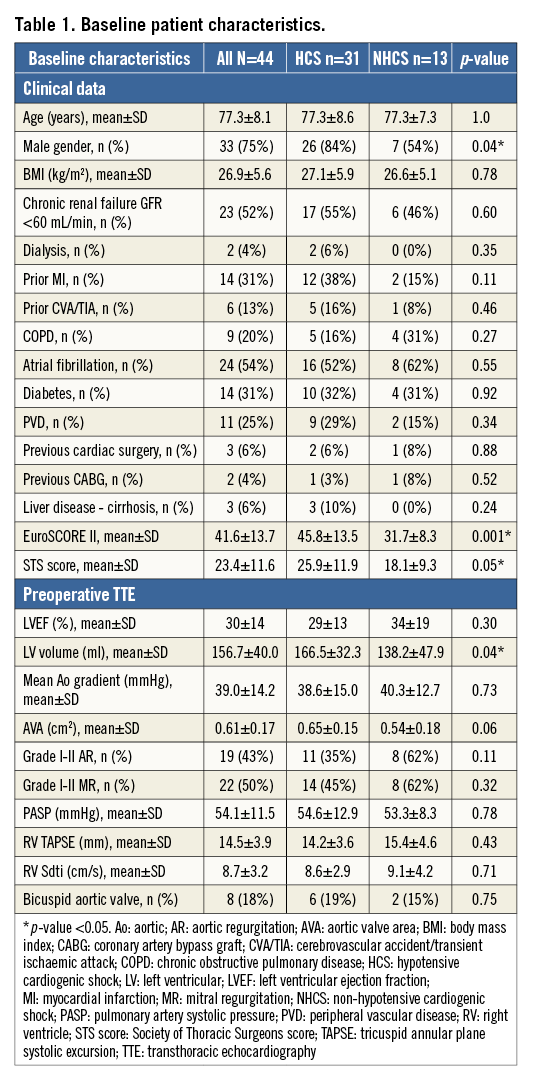
BASELINE PATIENT CHARACTERISTICS
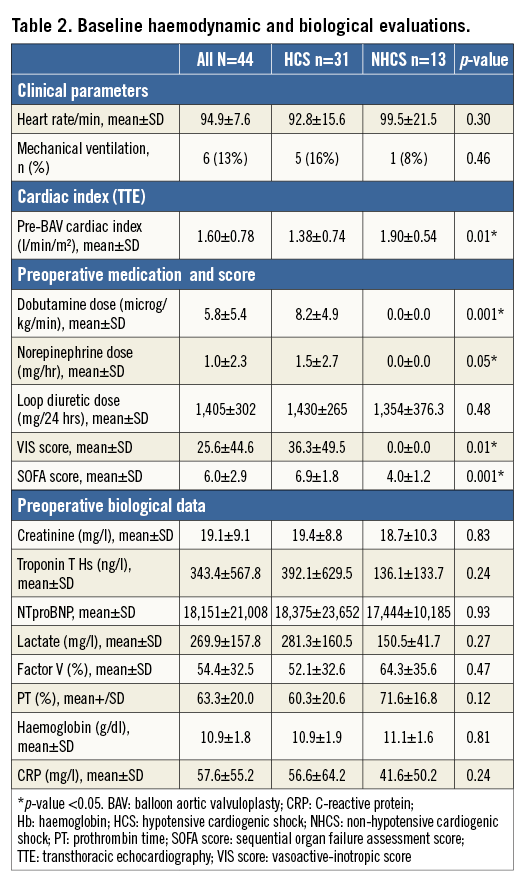
We identified 44 patients (around 15% of total BAV procedures in the two institutions) with either HCS (n=31, 84% male, mean age 77.3±8.6 years) or NHCS (n=13, 53% male, mean age 77.3±7.3 years), with a global mean EuroSCORE II of 41.6±13.7%. Baseline characteristics and comorbidities are presented in Table 1. Other indications for BAV in our two institutions (n=250) were (i) to assess the clinical response of a reduction in aortic gradient in borderline patients prior to consideration of definitive TAVR intervention (60%), (ii) as a bridge to TAVR because of a long waiting list (40%). Details about haemodynamic evaluation and peripheral injury can be found in the Supplementary Appendix and Table 2.
PROCEDURAL DATA
In the NHCS group, BAV was performed as soon as the diagnosis of non-hypotensive CS was established, 1.2±0.5 days after admission to the intensive care unit and before starting inotropes. In the HCS group, BAV was performed 4.1±2.9 days after admission to the intensive care unit and 3.2±3.8 days after the introduction of catecholamines. No patient received mechanical circulatory support.
Thirty-nine (88%) procedures were considered successful with a significant reduction of the aortic gradient. The immediate haemodynamic changes produced by BAV are shown in Supplementary Table 1. Postoperative TTE evaluation showed a significant overall decreased mean transaortic gradient (from 39.9±14.2 mmHg to 25.3±11.2 mmHg; p=0.01) and increased AVA (from 0.61±0.17 cm2 to 0.82±0.20 cm2; p=0.01).
OUTCOMES
i) In-hospital (Supplementary Table 2) and one-month outcomes (Supplementary Table 3)
Three patients (13%) died during the procedure (n=2 [6%] in the HCS subgroup and n=1 [7%] in the NHCS subgroup) due to a tamponade (n=1), severe aortic regurgitation (n=1), or haemodynamic collapse (n=1). Four patients (13%) with an efficient procedure were successfully weaned from inotropic support within the first 72 hours after the procedure in the hypotensive CS group. The total hospital mortality rate was 45% (n=20), n=16 in the HCS subgroup and n=4 in the NHCS subgroup (p=0.20). Twelve patients (27%) were discharged home before two weeks after BAV, n=4 (13%) in the HCS subgroup and n=8 (62%) in the NHCS subgroup (p=0.001). There were no major vascular complications or life-threatening bleedings, but n=3 (7%) major bleeding and n=2 (4%) minor bleeding. No patients were on dialysis at the time of BAV. Five patients (11%) required haemodialysis after the procedure (Supplementary Table 2). Intra-hospital mortality was significantly lower in patients with a successful procedure (n=13 [33%] vs. n=4 [80%]; p=0.04). Similarly, surviving patients had a lower post-procedural transaortic maximal velocity (2.9±0.9 m/s vs. 3.5±0.5 m/s; p=0.05) and a lower post-procedural transaortic mean gradient (22.5±9.7 vs. 30.5±12.6 mmHg; p=0.03).
A vasoactive-inotropic (VIS) score ≥10 (n=13 [76%] vs. n=8 [29%]; p=0.002) or a sequential organ failure assessment (SOFA) score ≥7 (n=15 [93%] vs. n=6 [21%]; p<0.001) was highly predictive of one-month mortality after BAV.
The mortality rate at one month reached 47% (n=21). Causes of death before and after one month are presented in Supplementary Table 4.
During the follow-up, n=12 (27%) patients had either a staged TAVR (n=10) or SAVR (n=2) with a median delay of 79±49 days after BAV, n=6 (19%) in the HCS group and n=6 (46%) in the NHCS group (p=0.06). Among the 12 patients who finally underwent TAVR or SAVR, n=4 (33%) were dead at one year. Follow-up after BAV for the entire cohort (n=44) is shown in Figure 1.
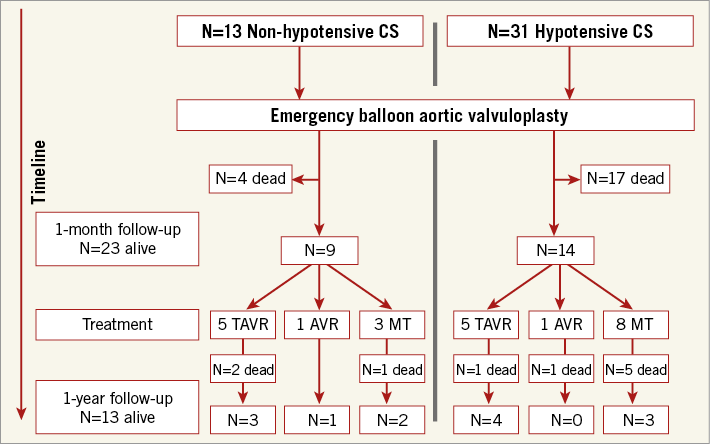
Figure 1. Follow-up after percutaneous balloon aortic valvuloplasty (BAV). AVR: aortic valve replacement; MT: medical treatment; TAVR: transcatheter aortic valve replacement
ii) One-year outcomes (Supplementary Table 3)
At one year, the rate of composite all-cause death or recurrent CS was 75% (n=33) and was significantly higher in the HCS subgroup (n=26 [83%] vs. n=7 [53%]; p=0.03). The mortality rate at one-year follow-up was 70% (n=31) in the overall cohort with a trend for better prognosis in the NHCS subgroup (mortality in HCS n=24 [77%] and in NHCS n=7 [53%]; p=0.11). One-year cumulative survival also showed a trend for better prognosis for the NHCS population with a log-rank p=0.09 (Figure 2).
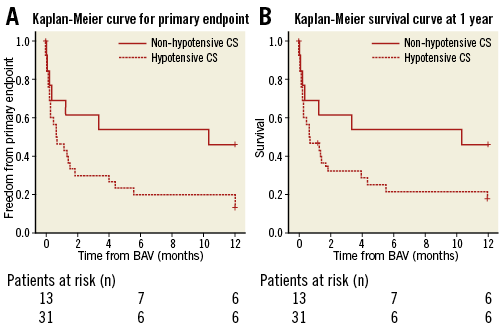
Figure 2. NHCS and HCS survival. A) Kaplan-Meier curve for the primary endpoint of patients undergoing BAV for hypotensive CS (HCS) and non-hypotensive CS (NHCS) due to severe aortic stenosis (log-rank p=0.05). B) Kaplan-Meier survival curve of patients undergoing BAV for hypotensive CS (HCS) and non-hypotensive CS (NHCS) due to severe aortic stenosis (log-rank p=0.09).
PREDICTIVE FACTORS OF THE PRIMARY ENDPOINT AND ONE-YEAR MORTALITY
In the global cohort, univariate predictive factors of the primary endpoint included a preoperative dose of dobutamine >5 microg/kg/min (100% vs. 57%; p=0.001) and delayed BAV >48 hrs (90% vs. 59%; p=0.01). Likewise, the predictive factors for one-year mortality were a preoperative dose of dobutamine >5 microg/kg/min (94% vs. 53%; p=0.02) and delayed BAV >48 hrs (86% vs. 54%; p=0.02). The prognosis of the three populations –NHCS, HCS with BAV ≤48 hrs and HCS with BAV >48 hrs– differed significantly at one-year follow-up regarding the primary endpoint (log-rank p=0.01) and survival (log-rank p=0.04) (Figure 3).
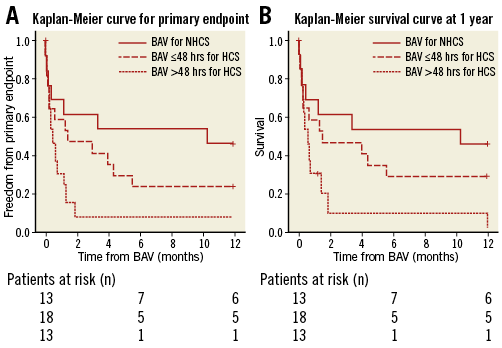
Figure 3. Timing of BAV. A) Kaplan-Meier curve for the primary endpoint of patients with BAV for NHCS, BAV ≤48 hrs for HCS, or BAV >48 hrs for HCS (log-rank p=0.01). B) Kaplan-Meier survival curve at one year for patients with BAV for NHCS, BAV ≤48 hrs for HCS, or BAV >48 hrs for HCS (log-rank p=0.04).
Discussion
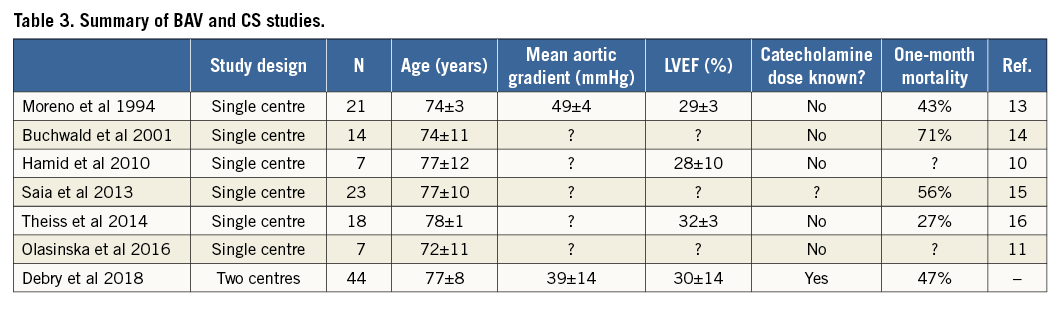
The present study, including 44 patients and conducted in two centres, is one of the largest published cohorts of patients undergoing BAV for cardiogenic shock related to a severe AS (Table 3). It discloses four key findings: (1) mortality at one year in contemporary patients remains high (70%), (2) mortality is directly related to the duration of shock before performing BAV, (3) more specifically, initiation of inotropic agents appears to be a critical time point, with a short time window (<48 hrs) after which conducting BAV becomes associated with a dire outcome, (4) the most favourable situation to perform BAV appears to be before the introduction of catecholamines, as it allows bringing 50% of patients to staged TAVR or AVR.
MORTALITY HAS NOT IMPROVED OVER THE YEARS
We have not seen any improvement regarding early mortality since 1994 (Table 3). In the modern era of TAVR, we confirm that BAV for CS remains associated with a dramatically poor prognosis, with only 27% of patients able to be treated by either TAVR or SAVR within the year, and 70% mortality at one year.
For the first time, we report that NHCS patients have a lower risk of death or recurrent CS at one year than the HCS subgroup (p=0.03) and that there is a trend towards lower mortality in that population at one month (p=0.14) and at one year (p=0.09). BAV may be useful for NHCS patients since it allows performing staged SAVR or TAVR in half of the cases.
Old studies included smaller cohorts of patients and details about haemodynamic monitoring were scarce. In particular, biological data regarding renal or hepatic failure and catecholamine doses at the time of valvuloplasty are lacking13,14. Even a recent study does not give much information about medical shock management and outcomes after BAV16. Trials exploring the outcomes of emergency TAVR for cardiogenic shock related to AS seem to have the same weakness22,23. Nevertheless, we report a one-month mortality rate of 47%, consistent with previous studies with in-hospital or one-month mortality varying from 43%13 to 71%14.
KEY ROLE OF THE TIME OF BAV RELATIVE TO INOTROPE SUPPORT
Our results illustrate the difficulty of improving the outcomes of patients in CS related to a severe AS. Duration of shock symptoms before causal treatment (to relieve valve obstruction), if attempted, seems to determine outcome. We show that the time of introduction of inotropic agents is crucial, since patients without amines (NHCS) have a better prognosis at one year. The valvuloplasty should be addressed as soon as signs of impaired end-organ perfusion appear, instead of starting inotropic agents.
When catecholamines have been initiated, a delay in performing BAV can be deadly. Performing an early BAV, during the first 48 hours, before for example increasing dobutamine dose beyond 5 microg/kg/min to maintain a systolic pressure above 90 mmHg, could be the answer to rapid deterioration of haemodynamic status. A delayed BAV after 48 hours may be useless, as hepatic and renal failure worsens. In a much smaller series (n=14), Buchwald et al also suggested a beneficial effect of an early BAV within the first 48 hours of shock diagnosis14.
IS IT TIME FOR 24 HRS A DAY “URGENT TAVR”?
Because one-year mortality remains high in AS patients with CS treated with BAV (47% in the present study), we can legitimately ask about the potential of emergency TAVR in the management of these patients. However, a retrospective study of patients (n=27) with AS and CS treated by emergency TAVR reported a 30-day and one-year mortality rate of 33% and 41%, respectively22. Another recent multicentre study investigating emergent TAVI and BAV (n=118) also showed high immediate procedural and 30-day mortality (33%), with more stroke and vascular complications for the TAVI group as compared to the BAV group17.
Altogether, this may suggest that emergent TAVR is not the ultimate option in AS patients with cardiogenic shock. Still, our data may suggest that to organise centres to perform emergent BAV in these patients would save lives.
Limitations
This was not a randomised trial and the low number of patients with cardiogenic shock prohibited multivariate analysis of factors impacting on 30-day survival. However, the selection of patients was very stringent and included an extensive screening (ECG, biology, echography and coronarography) allowing the exclusion of CS with uncertain aetiology or non-related to pure aortic stenosis. We believe that this very well characterised population may help to define predictive factors of mortality in this very difficult situation and thus help to define “when” and “for which patients” this procedure may be more beneficial. These data must be confirmed in large prospective multicentric cohorts.
Conclusions
Despite the initial success of urgent BAV, morbidity and mortality of CS related to severe AS remains dramatically high and is directly related to the duration of shock. Performing BAV before starting inotropic agents or within 48 hours of their initiation appears to be key to survival. With the improvement of the outcomes of TAVR, there is a need for a randomised trial comparing urgent BAV followed by staged TAVR and emergency TAVR for these unstable patients.
| Impact on daily practice Despite the initial success of urgent BAV, morbidity and mortality of CS related to severe AS remains dramatically high and is directly related to the duration of shock. Performing BAV before starting inotropic agents or within 48 hours of their initiation appears to be key to survival. |
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplementary data
Supplementary Appendix. Results.
Supplementary Table 1. BAV procedural data.
Supplementary Table 2. In-hospital outcomes according to VARC-2 criteria and postoperative data.
Supplementary Table 3. One-month and one-year outcomes.
Supplementary Table 4. Causes of death before and after one-month follow-up.
To read the full content of this article, please download the PDF.

