1 Preamble
Guidelines summarize and evaluate available evidence with the aim of assisting health professionals in proposing the best management strategies for an individual patient with a given condition. Guidelines and their recommendations should facilitate decision making of health professionals in their daily practice. However, the final decisions concerning an individual patient must be made by the responsible health professional(s) in consultation with the patient and caregiver as appropriate.
A great number of guidelines have been issued in recent years by the European Society of Cardiology (ESC) and its partners such as the European Association for Cardio-Thoracic Surgery (EACTS), as well as by other societies and organizations. Because of their impact on clinical practice, quality criteria for the development of guidelines have been established in order to make all decisions transparent to the user. The recommendations for formulating and issuing ESC Guidelines can be found on the ESC website (https://www.escardio.org/Guidelines). The ESC Guidelines represent the official position of the ESC on a given topic and are regularly updated.
In addition to the publication of Clinical Practice Guidelines, the ESC carries out the EURObservational Research Programme of international registries of cardiovascular diseases and interventions which are essential to assess diagnostic/therapeutic processes, use of resources and adherence to guidelines. These registries aim at providing a better understanding of medical practice in Europe and around the world, based on high-quality data collected during routine clinical practice.
The Members of this Task Force were selected by the ESC and EACTS, including representation from relevant ESC and EACTS sub-specialty groups, in order to represent professionals involved with the medical care of patients with this pathology. Selected experts in the field undertook a comprehensive review of the published evidence for management of a given condition according to ESC Clinical Practice Guidelines Committee (CPG). A critical evaluation of diagnostic and therapeutic procedures was performed, including assessment of the risk-benefit ratio. The level of evidence and the strength of the recommendation of particular management options were weighed and graded according to predefined scales, as outlined below.
The experts of the writing and reviewing panels provided declaration of interest forms for all relationships that might be perceived as real or potential sources of conflicts of interest. Their declarations of interest were reviewed according to the ESC declaration of interest rules and can be found on the ESC website (http://www.escardio.org/guidelines) and have been compiled in a report and published in a supplementary document simultaneously to the guidelines.
This process ensures transparency and prevents potential biases in the development and review processes. Any changes in declarations of interest that arise during the writing period were notified to the ESC and updated. The Task Force received its entire financial support from the ESC and EACTS without any involvement from the healthcare industry.
The ESC CPG supervises and coordinates the preparation of new guidelines. The Committee is also responsible for the endorsement process of these guidelines. The ESC Guidelines undergo extensive review by the CPG and external experts. After appropriate revisions the guidelines are signed-off by all the experts involved in the Task Force. The finalized document is signed-off by the CPG for publication in the European Heart Journal and the European Journal of Cardio-Thoracic Surgery. The guidelines were developed after careful consideration of the scientific and medical knowledge and the evidence available at the time of their dating.
The task of developing ESC/EACTS Guidelines also includes the creation of educational tools and implementation programmes for the recommendations including condensed pocket guideline versions, summary slides, summary cards for non-specialists and an electronic version for digital applications (smartphones, etc.). These versions are abridged and thus, for more detailed information, the user should always access to the full text version of the guidelines, which is freely available via the ESC and EACTS website and hosted on the EHJ and EJCTS website. The National Cardiac Societies of the ESC are encouraged to endorse, adopt, translate and implement all ESC Guidelines. Implementation programmes are needed because it has been shown that the outcome of disease may be favourably influenced by the thorough application of clinical recommendations.
Health professionals are encouraged to take the ESC/EACTS Guidelines fully into account when exercising their clinical judgment, as well as in the determination and the implementation of preventive, diagnostic or therapeutic medical strategies. However, the ESC/EACTS Guidelines do not override in any way whatsoever the individual responsibility of health professionals to make appropriate and accurate decisions in consideration of each patient's health condition and in consultation with that patient or the patient's caregiver where appropriate and/or necessary. It is also the healthcare professional's responsibility to verify the rules and regulations applicable in each country to drugs and devices at the time of prescription.
2 Introduction
2.1 WHY DO WE NEED NEW GUIDELINES ON VALVULAR HEART DISEASE?
Since the publication of the previous version of the guidelines on the management of valvular heart disease (VHD) in 2017, new evidence has accumulated, particularly on the following topics:
- Epidemiology: the incidence of the degenerative aetiology has increased in industrialized countries while, unfortunately, rheumatic heart disease is still too frequently observed in many parts of the world.123
- Current practices regarding interventions and medical management have been analysed in new surveys at the national and European level.
- Non-invasive evaluation using three-dimensional (3D) echocardiography, cardiac computed tomography (CCT), cardiac magnetic resonance (CMR), and biomarkers plays a more and more central role.
- New definitions of severity of secondary mitral regurgitation (SMR) based on the outcomes of studies on intervention.
- New evidence on anti-thrombotic therapies leading to new recommendations in patients with surgical or transcatheter bioprostheses for bridging during perioperative periods and over the long term. The recommendation for non- vitamin K antagonist oral anticoagulants (NOACs) was reinforced in patients with native valvular disease, except for significant mitral stenosis, and in those with bioprostheses.
- Risk stratification for the timing of intervention. This applies mostly to (i) the evaluation of progression in asymptomatic patients based on recent longitudinal studies mostly in aortic stenosis, and (ii) interventions in high-risk patients in whom futility should be avoided. Regarding this last aspect, the role of frailty is outlined.
- Results and indication of intervention:
- The choice of the mode of intervention: current evidence reinforces the critical role of the Heart Team, which should integrate clinical, anatomical, and procedural characteristics beyond conventional scores, and informed patient’s treatment choice.
- Surgery: increasing experience and procedural safety led to expansion of indications toward earlier intervention in asymptomatic patients with aortic stenosis, aortic regurgitation or mitral regurgitation and stress the preference for valve repair when it is expected to be durable. A particular emphasis is put on the need for more comprehensive evaluation and earlier surgery in tricuspid regurgitation.
- Transcatheter techniques: (i) Concerning transcatheter aortic valve implantation (TAVI), new information from randomized studies comparing TAVI vs. surgery in low-risk patients with a follow-up of 2 years has led to a need to clarify which types of patients should be considered for each mode of intervention. (ii) Transcatheter edge-to-edge repair (TEER) is increasingly used in SMR and has been evaluated against optimal medical therapy resulting in an upgrade of the recommendation. (iii) The larger number of studies on transcatheter valve-in-valve implantation after failure of surgical bioprostheses served as a basis to upgrade its indication. (iv) Finally, the encouraging preliminary experience with transcatheter tricuspid valve interventions (TTVI) suggests a potential role of this treatment in inoperable patients, although this needs to be confirmed by further evaluation.
The new evidence described above made a revision of the recommendations necessary.



2.2 METHODOLOGY
In preparation of the 2021 VHD Guidelines, a methodology group has been created for the first time, to assist the Task Force for the collection and interpretation of the evidence supporting specific recommendations. The group was constituted of two European Society of Cardiology (ESC) and two European Association for Cardio-Thoracic Surgery (EACTS) delegates who were also members of the Task Force. Although the principle activities of the group concerned the chapter on aortic stenosis and SMR, it was not limited to these two domains. The methodology group was at disposal, upon request of the Task Force members, to resolve specific methodological issues.
2.3 CONTENT OF THESE GUIDELINES
Decision making in VHD involves accurate diagnosis, timing of intervention, risk assessment and, based on these, selection of the most suitable type of intervention. These guidelines focus on acquired VHD, are oriented towards management, and do not deal with endocarditis,4 congenital valve disease5 (including pulmonary valve disease), or recommendations concerning sports cardiology and exercise in patients with cardiovascular disease,6 as separate guidelines have been published by the ESC on these topics.
2.4 NEW FORMAT OF THE GUIDELINES
The new guidelines have been adapted to facilitate their use in clinical practice and to meet readers' demands by focusing on condensed, clearly represented recommendations. At the end of the document, key points summarize the essentials. Gaps in evidence are listed to propose topics for future research. The guideline document will be harmonized with the chapter on VHD included in the ESC Textbook of Cardiovascular Medicine (ISBN: 9780198784906). The guidelines and the textbook are complementary. Background information and detailed discussion of the data that have provided the basis for the recommendations will be found in the relevant book chapter.
2.5 HOW TO USE THESE GUIDELINES
The Committee emphasizes that many factors ultimately determine the most appropriate treatment in individual patients within a given community. These factors include the availability of diagnostic equipment, the expertise of cardiologists and surgeons, especially in the field of valve repair and percutaneous intervention, and, notably, the wishes of well-informed patients. Furthermore, owing to the lack of evidence-based data in the field of VHD, most recommendations are largely the result of expert consensus opinion. Therefore, deviations from these guidelines may be appropriate in certain clinical circumstances.
3 General comments
This section defines and discusses concepts common to all the types of VHD including the Heart Team and Heart Valve Centres, the main evaluation steps of patients presenting with VHD, as well as the most commonly associated cardiac diseases.
3.1 CONCEPTS OF HEART TEAM AND HEART VALVE CENTRE
The main purpose of Heart Valve Centres as centres of excellence in the treatment of VHD is to deliver optimal quality of care with a patient-centred approach. The main requirements of a Heart Valve Centre are presented in Table 4.
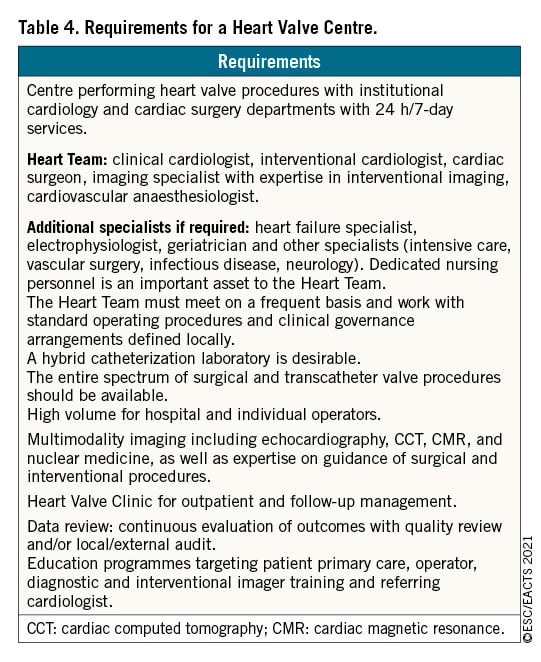
This is achieved through high procedural volume in conjunction with specialized training, continuous education, and focused clinical interest. Heart Valve Centres should promote timely referral of patients with VHD for comprehensive evaluation before irreversible damage occurs.
Decisions concerning treatment and intervention should be made by an active and collaborative Heart Team with expertise in VHD, comprising clinical and interventional cardiologists, cardiac surgeons, imaging specialists with expertise in interventional imaging,78 cardiovascular anaesthesiologists, and other specialists if necessary (e.g. heart failure specialists or electrophysiologists). Dedicated nursing personnel with expertise in the care of patients with VHD are also an important asset to the Heart Team. The Heart Team approach is particularly advisable for the management of high-risk and asymptomatic patients, as well as in case of uncertainty or lack of strong evidence.
Heart Valve Clinics are an important component of the Heart Valve Centres, aiming to provide standardized organization of care based on guidelines. Access to Heart Valve Clinics improves outcomes.9
Physicians experienced in the management of VHD and dedicated nurses organize outpatient visits, and referral to the Heart Team, if needed. Earlier referral should be encouraged if patient’s symptoms develop or worsen before the next planned visit.1011
Beside the whole spectrum of valvular interventions, expertise in interventional and surgical management of coronary artery disease (CAD), vascular diseases, and complications must be available.
Techniques with a steep learning curve may be performed with better results at hospitals with high procedural volume and experience. The relationship between case volume and outcomes for surgery and transcatheter interventions is complex but should not be denied.121314 However, the precise numbers of procedures per individual operator or hospital required to provide high-quality care remain controversial as inequalities exist between high- and middle-income countries.15 High-volume TAVI programmes are associated with lower mortality at 30 days, particularly at hospitals with a high surgical aortic valve replacement (SAVR) volume.1617 The data available on transcatheter mitral valve repair1418 and, even more so, transcatheter tricuspid procedures are more limited.
Since performance does not exclusively relate to intervention volume, internal quality assessment consisting of systematic recording of procedural data and patient outcomes at the level of a given Heart Valve Centre is essential, as well as participation in national or ESC/EACTS registries.
A Heart Valve Centre should have structured and possibly combined training programmes for interventionalists, cardiac surgeons, and imaging specialists131920 (https://ebcts.org/syllabus/). New techniques should be taught by competent mentors to minimize the effects of the learning curve.
Finally, Heart Valve Centres should contribute to optimizing the management of patients with VHD, provide corresponding services at the community level, and promote networks that include other medical departments, referring cardiologists and primary care physicians.
3.2 PATIENT EVALUATION
The aims of the evaluation of patients with VHD are to diagnose, quantify, and assess the mechanism of VHD, as well as its consequences.
3.2.1 CLINICAL EVALUATION
Precise evaluation of the patient's history and symptomatic status, and proper physical examination, in particular auscultation21 and search for heart failure signs, are crucial. In addition, assessment of their comorbidities and general condition require particular attention. The essential questions in the evaluation of a patient for valvular intervention are summarized in Figure 1 (Central illustration).
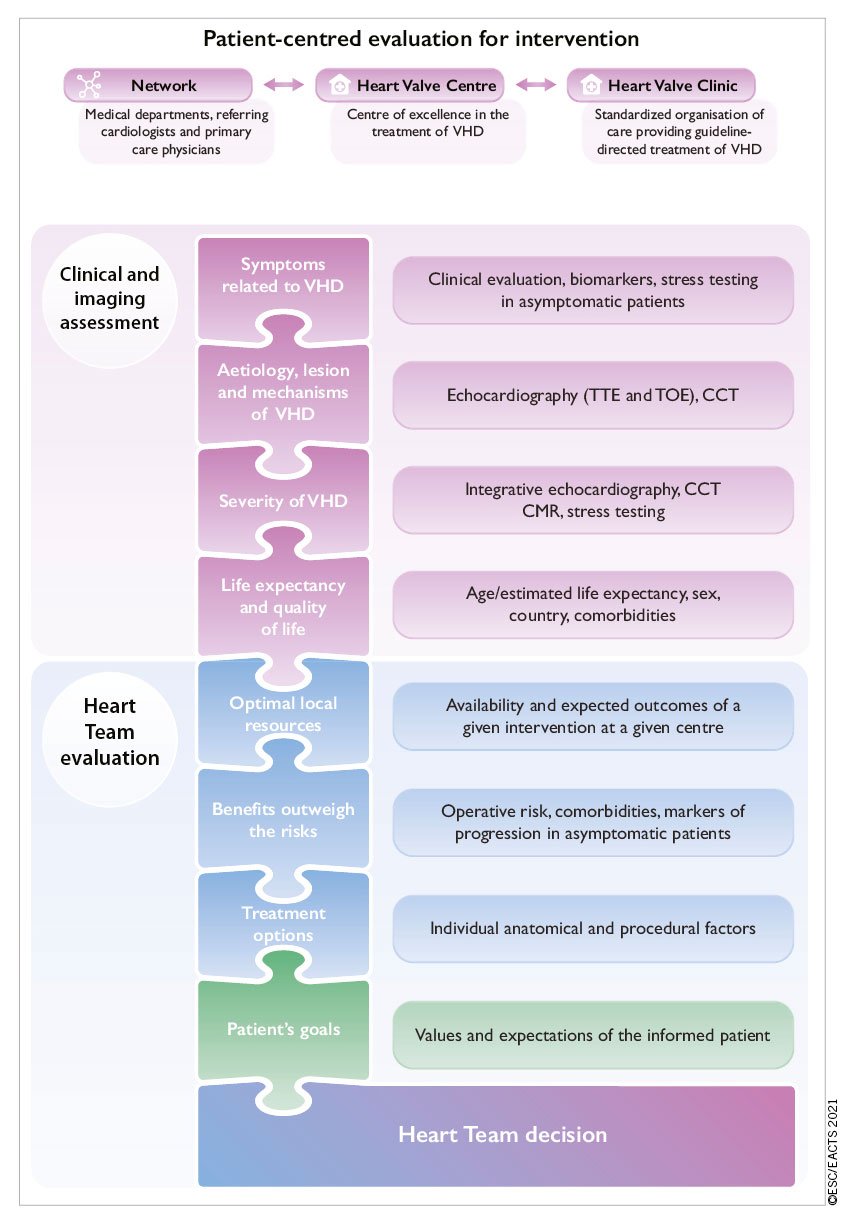
Figure 1. Central illustration: Patient-centred evaluation for intervention. VHD: valvular heart disease; CCT: cardiac computed tomography; CMR: cardiac magnetic resonance; TOE: transoesophageal echocardiography; TTE: transthoracic echocardiography.
3.2.2 ECHOCARDIOGRAPHY
Following adequate clinical evaluation, echocardiography is the key technique used to confirm the diagnosis of VHD, as well as to assess its aetiology, mechanisms, function, severity, and prognosis. It should be performed and interpreted by properly trained imagers.2223
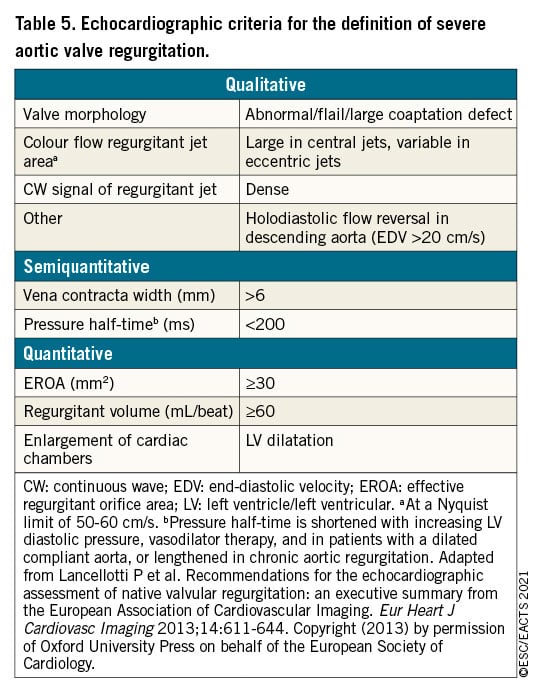
Echocardiographic criteria for the definition of severe valve stenosis and regurgitation are addressed in specific documents2425 and summarized in the specific sections of these guidelines. Echocardiography is also key to evaluating the feasibility of a specific intervention.
Indices of left ventricular (LV) enlargement and function are strong prognostic factors. Recent studies suggest that global longitudinal strain has greater prognostic value than LV ejection fraction (LVEF), although cut-off values are not uniform.2627 Transoesophageal echocardiography (TOE) should be considered when transthoracic echocardiography (TTE) is of suboptimal quality or when thrombosis, prosthetic valve dysfunction, or endocarditis is suspected. TOE is useful when detailed functional valve anatomy is required to assess repairability. Intraprocedural TOE, preferably 3D, is used to guide transcatheter mitral and tricuspid valve procedures and to assess the immediate result of surgical valve operations. Multimodality imaging may be required in specific conditions for evaluation and/or procedural guidance in TAVI and transcatheter mitral interventions.2829
3.2.3 OTHER NON-INVASIVE INVESTIGATIONS
3.2.3.1 Stress testing
The primary purpose of exercise testing is to unmask the objective occurrence of symptoms in patients who claim to be asymptomatic. It is especially useful for risk stratification in aortic stenosis.30 Exercise testing will also determine the level of recommended physical activity, including participation in sports. It should be emphasized that stress testing is safe and useful in asymptomatic patients with VHD. Unfortunately, the VHD II survey indicates that it is rarely performed in asymptomatic patients.1
Exercise echocardiography may identify the cardiac origin of dyspnoea. Prognostic impact has been shown mainly for aortic stenosis and mitral regurgitation.3132
The use of stress tests to detect CAD associated with severe valvular disease is discouraged because of their low diagnostic value and potential risks in symptomatic patients with aortic stenosis.
3.2.3.2 Cardiac magnetic resonance
In patients with inadequate echocardiographic quality or discrepant results, CMR should be used to assess the severity of valvular lesions, particularly regurgitant lesions, and to assess ventricular volumes, systolic function, abnormalities of the ascending aorta, and myocardial fibrosis.33 CMR is the reference method for the evaluation of right ventricular (RV) volumes and function and is therefore particularly useful to evaluate the consequences of tricuspid regurgitation.34 It also has an incremental value for assessing the severity of aortic and mitral regurgitation.
3.2.3.3 Computed tomography
CCT may contribute to the evaluation of valve disease severity, particularly in aortic stenosis3536 and possibly associated disease of the thoracic aorta (dilatation, calcification), as well as to evaluate the extent of MAC. CCT should be performed whenever the echocardiographic data indicate an aortic enlargement >40 mm, to clarify aortic diameter and to assess aortic morphology and configuration. CCT is essential in the pre-procedural planning of TAVI and can also be useful to assess patient-prosthesis mismatch (PPM).37 It is also a prerequisite for pre-procedural planning of mitral and tricuspid valve interventions.38 Positron emission tomography (PET)/CCT is useful in patients with a suspicion of endocarditis of a prosthetic valve.3940
3.2.3.4 Cinefluoroscopy
Cinefluoroscopy is particularly useful for assessing the kinetics of the leaflet occluders of a mechanical prosthesis.
3.2.3.5 Biomarkers
B-type natriuretic peptide (BNP) serum levels, corrected for age and sex, are useful in asymptomatic patients and may assist selection of the appropriate time point for a given intervention,41 particularly if the level rises during follow-up. Other biomarkers have been tested, with evidence for fibrosis, inflammation, and adverse ventricular remodelling, which could improve decision making.42
3.2.3.6 Multimarkers and staging
In patients with at least moderate aortic stenosis and LVEF >50%, staging according to damage associated with aortic stenosis on LV/RV, left atrium (LA), mitral /tricuspid valve, and pulmonary circulation was predictive of excess mortality after TAVI and SAVR, and may help to identify patients who will benefit from an intervention.4344
3.2.4 INVASIVE INVESTIGATIONS
3.2.4.1 Coronary angiography
Coronary angiography is recommended for the assessment of CAD when surgery or an intervention is planned, to determine if concomitant coronary revascularization is recommended (see recommendations for management of CAD in patients with VHD).4546 Alternatively, owing to its high negative predictive value, CCT may be used to rule out CAD in patients who are at low risk of atherosclerosis. The usefulness of fractional flow reserve or instantaneous wave-free ratio in patients with VHD is not well established, and caution is warranted in the interpretation of these measurements when VHD, and in particular aortic stenosis, is present.4748
3.2.4.2 Cardiac catheterization
The measurement of pressures and cardiac output or the assessment of ventricular performance and valvular regurgitation by ventricular angiography or aortography is restricted to situations where non-invasive evaluation by multimodality imaging is inconclusive or discordant with clinical findings. When elevated, pulmonary pressure is the only criterion to support the indication for surgery, and confirmation of echo data by invasive measurement is recommended. Right heart catheterization is also indicated in patients with severe tricuspid regurgitation as Doppler gradient may be impossible or underestimate the severity of pulmonary hypertension.
3.2.5 ASSESSMENT OF COMORBIDITY
The choice of specific examinations to assess comorbidity is guided by the clinical evaluation.
3.3 RISK STRATIFICATION
Risk stratification applies to any sort of intervention and is required for weighing the risk of intervention against the expected natural history of VHD and for choosing the type of intervention. Most experience relates to surgery and TAVI.
3.3.1 RISK SCORES
The Society of Thoracic Surgeons (STS) predicted risk of mortality (PROM) score (http://riskcalc.sts.org/stswebriskcalc/calculate) and the European System for Cardiac Operative Risk Evaluation II (EuroSCORE II; http://www.euroscore.org/calc.html) accurately discriminate high- and low-risk surgical patients and show good calibration to predict postoperative outcome after valvular surgery in the majority of the patients,4950 while risk estimation may be less accurate in high-risk patients.51 The STS-PROM score is dynamic and changes over time. Of note, the risk scores have not been validated for isolated tricuspid surgical interventions.
In isolation, surgical scores have major limitations for practical use in patients undergoing transcatheter intervention because they do not include major risk factors such as frailty, as well as anatomical factors with impact on the procedure, either surgical or transcatheter [porcelain aorta, previous chest radiation, mitral annular calcification (MAC)].
New scores have been developed to estimate the risk in patients undergoing TAVI, with better accuracy and discrimination than the surgical risk scores, despite numerous limitations525354 (Supplementary Table 1).
Experience with risk stratification is currently limited for other interventional procedures, such as mitral or tricuspid interventions.
3.3.2 OTHER FACTORS
Other factors should be taken into account:
• Frailty, defined as a decrease of physiologic reserve and ability to maintain homeostasis leading to an increased vulnerability to stresses and conferring an increased risk of morbidity and mortality after both surgery and TAVI.55 The assessment of frailty should not rely on a subjective approach, such as the ‘eyeball test’, but rather on a combination of different objective estimates.5556575859 Several tools are available for assessing frailty (Supplementary Table 2,59 and Supplementary Table 3).60
• Malnutrition61 and cognitive dysfunction62 both predict poor prognosis.
• Other major organ failures (Supplementary Table 4), in particular the combination of severe lung disease,6364 postoperative pain from sternotomy or thoracotomy and prolonged time under anaesthesia in patients undergoing SAVR via full sternotomy, may contribute to pulmonary complications. There is a positive association between the impairment of renal function and increased mortality after valvular surgery and transcatheter procedures,65 especially when the glomerular filtration rate is <30 mL/min. Liver disease, is also an important prognostic factor.66
• Anatomical aspects affecting procedural performance such as porcelain aorta or severe MAC67 (see Table 6 in section 5.1.3, and Supplementary Figure 1).
At the extreme of the risk spectrum, futility should be avoided. Therapeutic futility has been defined as a lack of medical efficacy, particularly when the physician judges that the therapy is unlikely to produce its intended clinical results, or lack of meaningful survival according to the personal values of the patient. Assessment of futility goes beyond survival and includes functional recovery. The futility of interventions has to be taken into consideration, particularly for transcatheter interventions.63
The high prevalence of comorbidity in the elderly makes assessment of the risk/benefit ratios of interventions more difficult, therefore the role of the Heart Team is essential in this specific population of patients (Supplementary Table 5).
3.4 PATIENT-RELATED ASPECTS
Patient-related life expectancy and expected quality of life should be considered. The patient and their family should be thoroughly informed and assisted in their decision on the best treatment option.13 A patient-centred approach would take patient-reported outcome measures and patient-reported experience measures into consideration and make these parameters part of the informed choice offered to patients.6869
When benefit in symptom relief aligns with a patient’s goals, care is not futile. However, care is futile when no life prolongation or symptom relief is anticipated.70
3.5 LOCAL RESOURCES
Even if it is desirable that Heart Valve Centres are able to perform a large spectrum of procedures, either surgical or catheter-based, specialization and thereby expertise in specific domains will vary and should be taken into account when deciding on the orientation of the patient in specific cases, such as complex surgical valve repair or transcatheter intervention.
In addition, penetration of transcatheter interventions is heterogeneous worldwide and highly dependent on socioeconomic inequalities.1571 Appropriate stewardship of economic resources is a fundamental responsibility of the Heart Team.
3.6 MANAGEMENT OF ASSOCIATED CONDITIONS
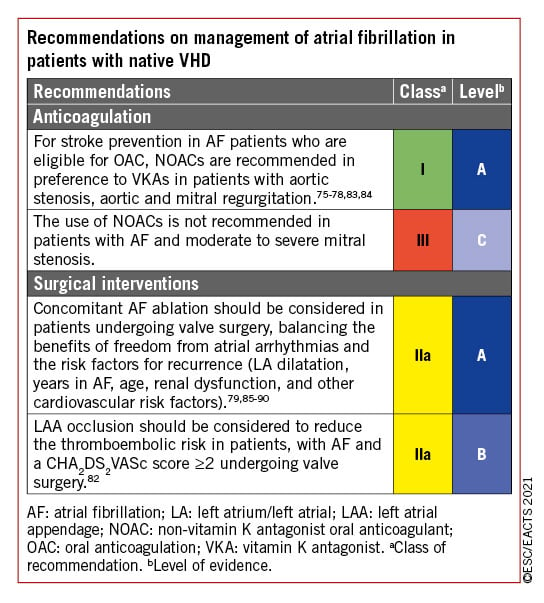
3.6.1 CORONARY ARTERY DISEASE
Recommendations for the management of CAD associated with VHD are provided below and are detailed in specific sections (section 5 and section 6.2) of this guideline document, as well as in other dedicated guideline documents.45467273
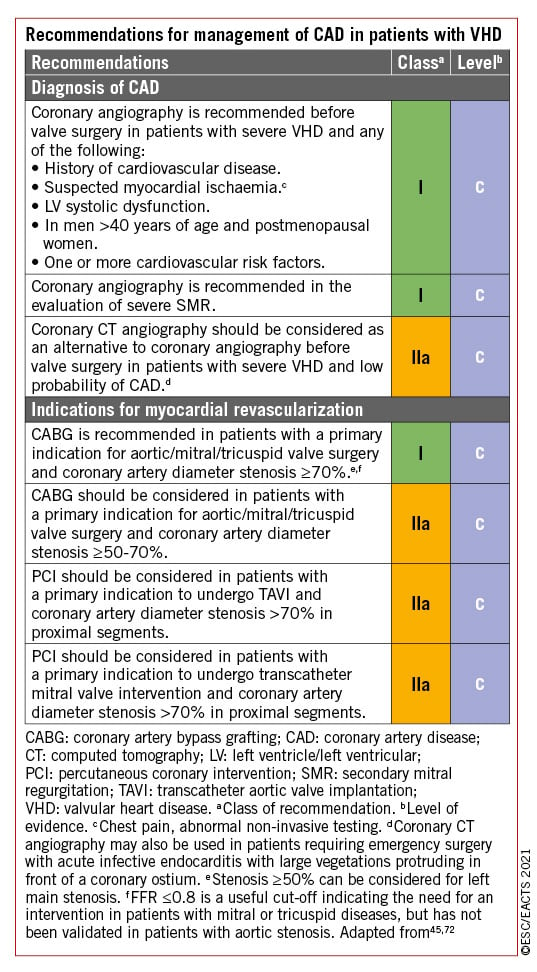
3.6.2 ATRIAL FIBRILLATION
Detailed recommendations on the management of patients with atrial fibrillation (AF) including management of anticoagulation are provided in specific guidelines.74 NOACs are recommended in patients with aortic stenosis, aortic regurgitation or mitral regurgitation presenting with AF75767778 as subgroup analyses of randomized controlled trials (RCTs) support the use of apixaban, dabigatran, edoxaban, and rivaroxaban. The use of NOACs is not recommended in patients who have AF associated with clinically significant mitral stenosis or those with mechanical prostheses.
Surgical ablation of AF combined with mitral valve surgery effectively reduces the incidence of AF but has no impact on adjusted short-term survival. An increased rate of pacemaker implantation has been observed after surgical ablation (9.5%, vs. 7.6% in the group with AF and no surgical ablation).79 Concomitant AF ablation should be considered in patients undergoing cardiac surgery, balancing the benefits of freedom from atrial arrhythmias with the risk factors for recurrence, such as age, LA dilatation, years in AF, renal dysfunction, and other cardiovascular risk factors. In addition, left atrial appendage (LAA) occlusion should be considered in combination with valve surgery in patients with AF and a CHA2DS2VASc score ≥2 to reduce the thromboembolic risk.808182 The selected surgical technique should ensure complete occlusion of the LAA. For patients with AF and risk factors for stroke, long-term oral anticoagulation (OAC) is currently recommended, irrespective of the use of surgical ablation of AF and/or surgical LAA occlusion.
Recommendations for the management of AF in native VHD are summarized in the following table. The recommendations concerning patients with valve prostheses, and the combination of anticoagulants and antiplatelet agents in patients undergoing PCI, are described in section 11 (section 11.3.2.2 and related table of recommendations for perioperative and postoperative antithrombotic management of valve replacement or repair).
3.7 ENDOCARDITIS PROPHYLAXIS
Antibiotic prophylaxis should be considered for high-risk procedures in patients with prosthetic valves, including transcatheter valves, or with repairs using prosthetic material, and in patients with previous episode(s) of infective endocarditis.4 Particular attention to dental and cutaneous hygiene and strict aseptic measures during any invasive procedure are advised in this population. Antibiotic prophylaxis should be considered in dental procedures involving manipulation of the gingival or periapical region of the teeth or manipulation of the oral mucosa.4
3.8 PROPHYLAXIS FOR RHEUMATIC FEVER
Prevention of rheumatic heart disease should preferably target the first attack of acute rheumatic fever. Antibiotic treatment of group A Streptococcus infection throat is key in primary prevention. Echocardiographic screening in combination with secondary antibiotic prophylaxis in children with evidence of latent rheumatic heart disease is currently investigated to reduce its prevalence in endemic regions.91 In patients with established rheumatic heart disease, secondary long-term prophylaxis against rheumatic fever is recommended: benzathine benzyl penicillin 1.2 MUI every 3 to 4 weeks over 10 years. Lifelong prophylaxis should be considered in high-risk patients according to the severity of VHD and exposure to group A Streptococcus.92939495
4 Aortic regurgitation
Aortic regurgitation can be caused by primary disease of the aortic valve cusps and/or abnormalities of the aortic root and ascending aortic geometry. Degenerative tricuspid and bicuspid aortic regurgitation are the most common aetiologies in high-income countries, accounting for approximately two-thirds of the underlying aetiology of aortic regurgitation in the EURObservational Registry Programme Valvular Heart Disease II registry.1 Other causes include infective and rheumatic endocarditis. Acute severe aortic regurgitation is mostly caused by infective endocarditis, and less frequently by aortic dissection.
4.1 EVALUATION
4.1.1 ECHOCARDIOGRAPHY
Echocardiography is the key examination used to describe valve anatomy, quantify aortic regurgitation, evaluate its mechanisms, define the morphology of the aorta, and determine the feasibility of valve-sparing aortic surgery or valve repair.9697 Identification of the mechanism follows the same principle such as for mitral regurgitation: normal cusps but insufficient coaptation due to dilatation of the aortic root with central jet (type 1), cusp prolapse with eccentric jet (type 2), or retraction with poor cusp tissue quality and large central or eccentric jet (type 3).96 Quantification of aortic regurgitation follows an integrated approach considering qualitative, semi-quantitative, and quantitative parameters2498 (Table 5). New parameters obtained by 3D echocardiography and two-dimensional (2D) strain imaging as LV global longitudinal strain may be useful, particularly in patients with borderline LVEF where they may help in the decision for surgery.99 Measurement of the aortic root and ascending aorta in 2D is performed at four levels: annulus, sinuses of Valsalva, sinotubular junction, and tubular ascending aorta.100101 Measurements are performed in the parasternal long-axis view from leading edge to leading edge at end diastole, except for the aortic annulus, which is measured in mid systole. As it will have surgical consequences, it is important to differentiate three phenotypes of the ascending aorta: aortic root aneurysms (sinuses of Valsalva >45 mm), tubular ascending aneurysm (sinuses of Valsalva <40-45 mm), and isolated aortic regurgitation (all aortic diameters <40 mm). The calculation of indexed values to account for body size has been suggested,102 in particular in patients with small stature. Anatomy of the aortic valve cusps and its suitability for valve repair should be provided by preoperative TOE if aortic valve repair or a valve-sparing surgery of the aortic root is considered. Intraoperative evaluation of the surgical result by TOE is mandatory in patients undergoing aortic valve preservation or repair.
4.1.2 COMPUTED TOMOGRAPHY AND CARDIAC MAGNETIC RESONANCE
CMR should be used to quantify the regurgitant fraction when echocardiographic measurements are equivocal or discordant with clinical findings. In patients with aortic dilatation, CCT is recommended to assess the maximum diameter at four levels, as in echocardiography. CMR can be used for follow-up, but indication for surgery should preferably be based on CCT measurements. Different methods of aortic measurements have been reported. To improve reproducibility, it is recommended to measure diameters using the inner-inner-edge technique at end diastole on the strictly transverse plane by double oblique reconstruction perpendicular to the axis of blood flow of the corresponding segment. Maximum root diameter should be taken from sinus-to-sinus diameter rather than sinus-to-commissure diameter, as it correlates more closely to long-axis leading-edge-to-leading-edge echo maximum diameters.103104
4.2 INDICATIONS FOR INTERVENTION
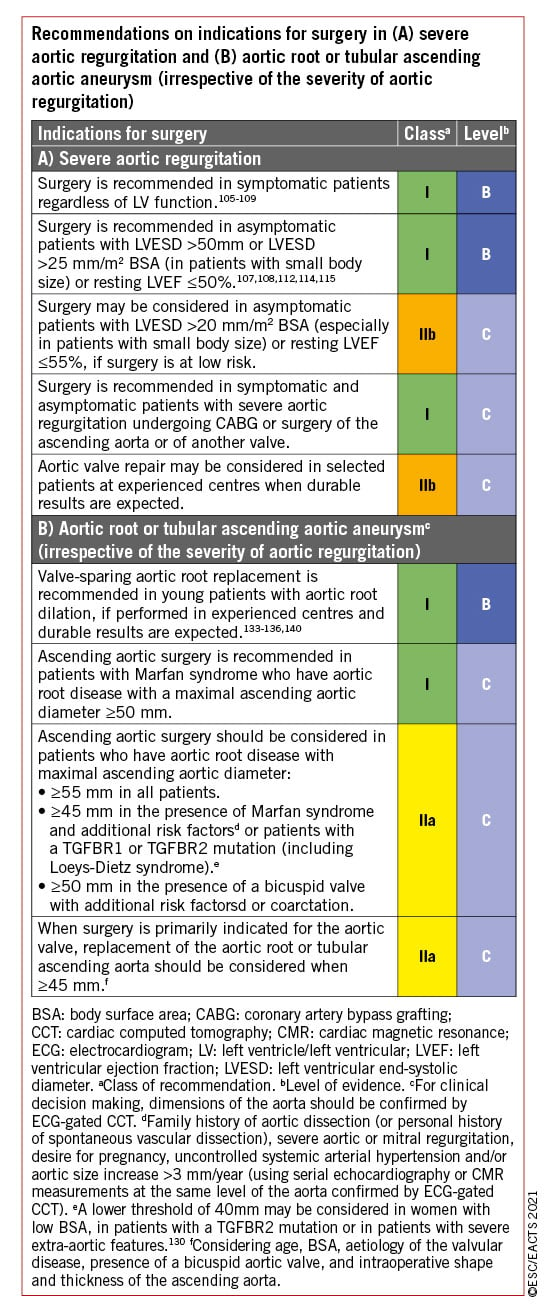
Acute aortic regurgitation may require urgent surgery. It is mainly caused by infective endocarditis and aortic dissection but may also occur after blunt chest trauma and iatrogenic complications during catheter-based cardiac interventions. Specific guidelines deal with these entities.4101 The recommendations on indications for surgery in severe aortic regurgitation and aortic root disease may be related to symptoms, status of the LV, or dilatation of the aorta [see table of recommendations on indications for surgery in severe aortic regurgitation and aortic root or tubular ascending aortic aneurysm (irrespective of the severity of aortic regurgitation), and Figure 2].
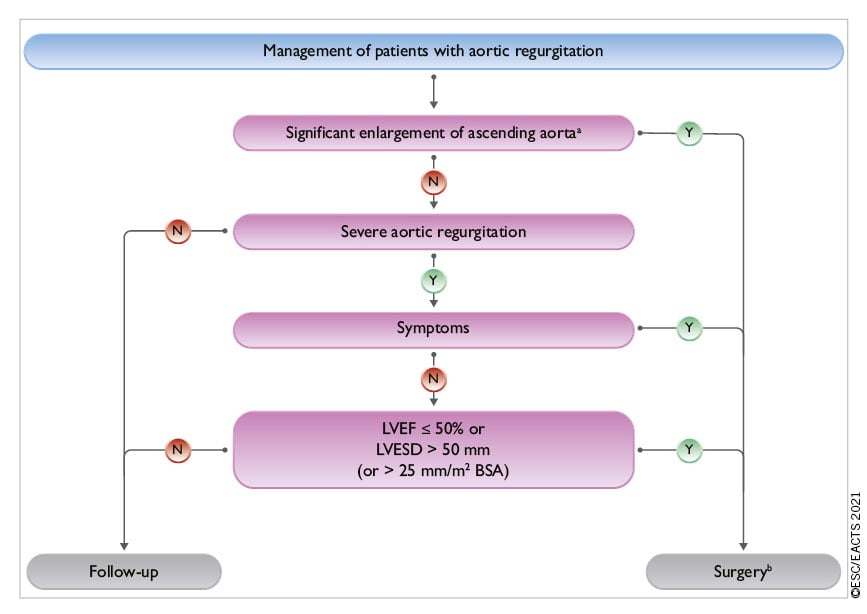
Figure 2. Management of patients with aortic regurgitation. BSA: body surface area; LV: left ventricle/left ventricular; LVESD: left ventricle end-systolic diameter; LVEF: left ventricular ejection fraction. aSee recommendations on indications for surgery in severe aortic regurgitation and aortic root disease for definition. bSurgery should also be considered if significant changes in LV or aortic size occur during follow-up.
In symptomatic patients, surgery is recommended irrespective of the LVEF as long as aortic regurgitation is severe and the operative risk is not prohibitive.105106107108109 Surgery is recommended in symptomatic and asymptomatic patients with severe aortic regurgitation undergoing coronary artery bypass grafting (CABG), or surgery of the ascending aorta or another valve.110111 In asymptomatic patients with severe aortic regurgitation, impairment of LV function [LVEF ≤50% or left ventricular end-systolic diameter (LVESD) >50 mm] are associated with worse outcomes and surgery should therefore be pursued when these cut-offs are reached.107108112113114 LVESD should be related to body surface area (BSA) and a cut-off of 25 mm/m2 BSA appeared to be more appropriate, especially in patients with small body size (BSA <1.68 m2) or with large BSA who are not overweight.108115 Some recent retrospective, non-randomized studies emphasized the role of indexed LVESD and proposed a lower cut-off value of 20 or 22 mm/m² BSA for the indexed LVESD.116117118 One of these studies also suggests a higher cut-off value of 55% for LVEF.118 Based on these data, low-risk surgery may be discussed in some selected asymptomatic patients with LVESD >20 mm/m2 or resting LVEF between 50% and 55%. In patients not reaching the thresholds for surgery, close follow-up is needed, and exercise testing should be liberally performed to identify borderline symptomatic patients. Progressive enlargement of the LV, or a progressive decrease in its function in asymptomatic patients not reaching the thresholds for surgery but with significant LV dilatation [left ventricular end-diastolic diameter (LVEDD) >65 mm], may also be an appropriate indicator for timing operations in asymptomatic patients.
TAVI may be considered in experienced centres for selected patients with aortic regurgitation and ineligible for SAVR.119120
In patients with a dilated aorta, the rationale for surgery has been best defined in patients with Marfan syndrome and root dilation.121122 Root aneurysms require root replacement, with or without preservation of the native aortic valve. In contrast, tubular ascending aortic aneurysms in the presence of normal aortic valves require only a supracommissural tube graft replacement. In patients with aortic diameters borderline indicated for aortic surgery, the family history, age, and anticipated risk of the procedure should be taken into consideration. Irrespective of the degree of aortic regurgitation and type of valve pathology, in patients with an aortic diameter ≥55 mm with tricuspid or bicuspid aortic valves, ascending aortic surgery is recommended (see recommendations on indications for surgery in severe aortic regurgitation and aortic root disease) when the operative risk is not prohibitive.123124125 In individuals with bicuspid aortic valve, when additional risk factors or coarctation126 are present, surgery should be considered when aortic diameter is ≥50 mm.127128129 In all patients with Marfan syndrome, aortic surgery is recommended for a maximal aortic diameter ≥50 mm.5121122 When additional risk factors are present in patients with Marfan syndrome and in patients with a TGFBR1 or TGFBR2 mutation (including Loeys-Dietz syndrome), surgery should be considered at a maximal aortic diameter ≥45 mm121130 and even earlier (aortic diameter of 40 mm or more) in women with low BSA, patients with a TGFBR2 mutation, or patients with severe extra-aortic features that appear to be at particularly high risk.130 For patients who have an indication for aortic valve surgery, an aortic diameter ≥45 mm is considered to indicate concomitant surgery of the aortic root or tubular ascending aorta. The patient's stature, the aetiology of the valvular disease (bicuspid valve), and the intraoperative shape and wall thickness of the ascending aorta should be considered for individual decisions.
The choice of the surgical procedure should be adapted according to the experience of the team, the presence of an aortic root aneurysm, characteristics of the cusps, life expectancy, and desired anticoagulation status.
Valve replacement is the standard procedure in the majority of patients with aortic regurgitation. Aortic valve-sparing root replacement and valve repair yield good long-term results in selected patients, with low rates of valve-related events as well as good quality of life131132133134135136137138139140 when performed in experienced centres. Aortic valve-sparing root replacement is recommended in younger patients who have an enlargement of the aortic root with normal cusp motion, when performed by experienced surgeons.133134135136140 In selected patients, aortic valve repair132137 or the Ross procedure138139 may be an alternative to valve replacement, when performed by experienced surgeons.
4.3 MEDICAL THERAPY
Medical therapy, especially angiotensin-converting enzyme inhibitors (ACEI) or dihydropiridines, may provide symptomatic improvement in individuals with chronic severe aortic regurgitation in whom surgery is not feasible. The value of ACEI or dihydropiridine in delaying surgery in the presence of moderate or severe aortic regurgitation in asymptomatic patients has not been established and their use is not recommended for this indication.
In patients who undergo surgery but continue to suffer from heart failure or hypertension, ACEI, angiotensin receptor blockers (ARBs), and beta-blockers are useful.141142
In patients with Marfan syndrome, beta-blockers remain the mainstay for medical treatment and reducing shear stress and aortic growth rate and should be considered before and after surgery.143144145 While ARBs did not prove to have a superior effect when compared to beta-blockers, they may be considered as an alternative in patients intolerant to beta-blockers.146147148 By analogy, while there are no studies that provide supporting evidence, it is common clinical practice to advise beta-blocker or ARBs in patients with bicuspid aortic valve if the aortic root and/or ascending aorta is dilated. Management of aortic regurgitation during pregnancy is discussed in section 13.
4.4 SERIAL TESTING
All asymptomatic patients with severe aortic regurgitation and normal LV function should be followed up at least every year. In patients with either a first diagnosis or with LV diameter and/or ejection fraction showing significant changes or approaching thresholds for surgery, follow-up should be continued at 3-6-month intervals. Surgery may be considered in asymptomatic patients with significant LV dilatation (LVEDD >65 mm), and with progressive enlargement in the size of LV or progressive decrease of LVEF during follow-up. Patient’s BNP levels could be of potential interest as a predictor of outcomes (particularly symptom onset and deterioration of LV function) and may be helpful in the follow-up of asymptomatic patients.149 Patients with mild-to-moderate aortic regurgitation can be seen on a yearly basis and echocardiography performed every 2 years.
If the ascending aorta is dilated (>40 mm), it is recommended to systematically perform CCT or CMR. Follow-up assessment of the aortic dimension should be performed using echocardiography and/or CMR. Any increase >3 mm should be validated by CCT angiography/CMR and compared with baseline data. After repair of the ascending aorta, Marfan patients remain at risk for dissection of the residual aorta and lifelong regular multidisciplinary follow-up at an expert centre is required.
4.5 SPECIAL PATIENT POPULATIONS
If aortic regurgitation requiring surgery is associated with severe primary and secondary mitral regurgitation, both should be treated during the same operation.
In patients with moderate aortic regurgitation who undergo CABG or mitral valve surgery, the decision to treat the aortic valve is controversial, as data show that progression of moderate aortic regurgitation is very slow in patients without aortic dilation.150 The Heart Team should decide based on the aetiology of aortic regurgitation, other clinical factors, the life expectancy of the patient, and the patient's operative risk.
The level of physical and sports activity in the presence of a dilated aorta remains a matter of clinical judgment in the absence of evidence. Current guidelines are very restrictive, particularly regarding isometric exercise, to avoid a catastrophic event.151 This approach is justified in the presence of connective tissue disease, but a more liberal approach is likely to be appropriate in other patients.
Given the familial risk of thoracic aortic aneurysms, screening and referral for genetic testing of the patient's first-degree relatives with appropriate imaging studies is indicated in patients with connective tissue disease. For patients with bicuspid valves, it is appropriate to have an echocardiographic screening of first-degree relatives.
5 Aortic stenosis
Aortic stenosis is the most common primary valve lesion requiring surgery or transcatheter intervention in Europe1 and North America. Its prevalence is rising rapidly as a consequence of the ageing population.2152
5.1 EVALUATION
5.1.1 ECHOCARDIOGRAPHY
Echocardiography is key to confirming the diagnosis and severity of aortic stenosis, assessing valve calcification, LV function and wall thickness, detecting other valve disease or aortic pathology, and providing prognostic information.43153154 Assessment should be undertaken when blood pressure (BP) is well controlled to avoid the confounding flow effects of increased afterload. New echocardiographic parameters, stress imaging and CCT provide important adjunctive information when severity is uncertain (Figure 3).
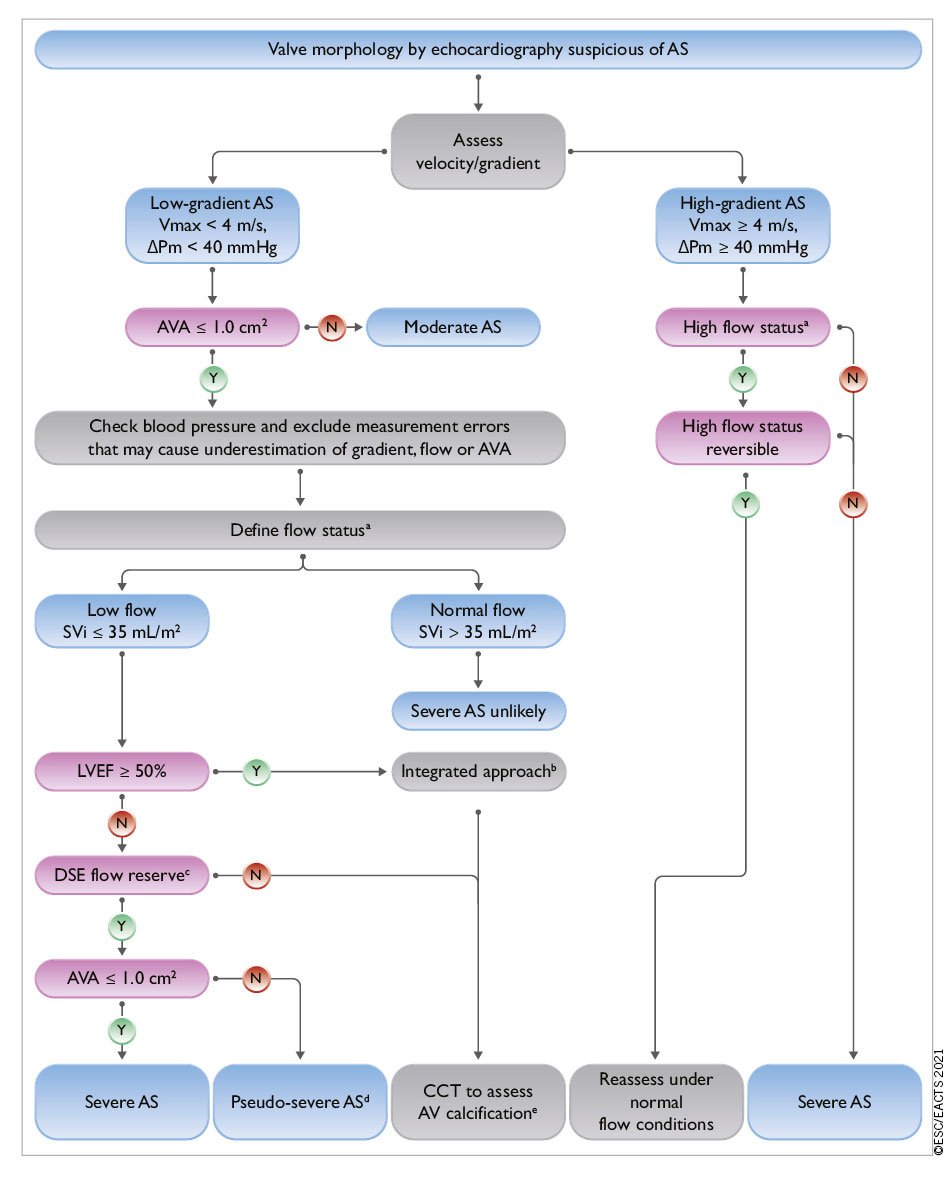
Figure 3. Integrated imaging assessment of aortic stenosis. AS: aortic stenosis; AV: aortic valve; AVA: aortic valve area; CT: computed tomography; ΔPm: mean pressure gradient; DSE: dobutamine stress echocardiography; LV: left ventricle/left ventricular; LVEF: left ventricular ejection fraction; SVi: stroke volume index; Vmax: peak transvalvular velocity. aHigh flow may be reversible in patients with anaemia, hyperthyroidism or arterio-venous fistulae, and may also be present in patients with hypertrophic obstructive cardiomyopathy. Upper limit of normal flow using pulsed Doppler echocardiography: cardiac index 4.1 L/min/m2 in men and women, SVi 54 mL/m2 in men, 51 mL/m2 in women).155 bConsider also: typical symptoms (with no other explanation), LV hypertrophy (in the absence of coexistent hypertension) or reduced LV longitudinal function (with no other cause). cDSE flow reserve: >20% increase in stroke volume in response to low-dose dobutamine. dPseudo-severe aortic stenosis: AVA >1.0 cm2 with increased flow. eThresholds for severe aortic stenosis assessed by means of CT measurement of aortic valve calcification (Agatston units): men >3000, women >1600: highly likely; men >2000, women >1200: likely; men <1600, women <800: unlikely.
Current international recommendations for the echocardiographic evaluation of patients with aortic stenosis25 depend upon measurement of mean pressure gradient (the most robust parameter), peak transvalvular velocity (Vmax), and valve area. Although valve area is the theoretically ideal measurement for assessing severity, there are numerous technical limitations. Clinical decision making in discordant cases should therefore take account of additional parameters: functional status, stroke volume, Doppler velocity index,156 degree of valve calcification, LV function, the presence or absence of LV hypertrophy, flow conditions, and the adequacy of BP control.25 Low flow is arbitrarily defined by a stroke volume index (SVi) ≤35 mL/m² –a threshold that is under current debate.155157158 The use of sex -specific thresholds has been recently proposed.159 Four broad categories can be defined:
- High-gradient aortic stenosis [mean gradient ≥40 mmHg, peak velocity ≥4.0 m/s, valve area ≤1 cm2 (or ≤0.6 cm²/m²)]. Severe aortic stenosis can be assumed irrespective of LV function and flow conditions.
- Low-flow, low-gradient aortic stenosis with reduced ejection fraction (mean gradient <40 mmHg, valve area ≤1 cm2, LVEF <50%, SVi ≤35 mL/m2). Low-dose dobutamine stress echocardiography (DSE) is recommended to distinguish between true severe and pseudo-severe aortic stenosis (increase in valve area to >1.0 cm2 with increased flow) and identify patients with no flow (or contractile) reserve.160 However, utility in elderly patients has only been evaluated in small registries.161
- Low-flow, low-gradient aortic stenosis with preserved ejection fraction (mean gradient <40 mmHg, valve area ≤1 cm2, LVEF ≥50%, SVi ≤35 mL/m2). Typically encountered in hypertensive elderly subjects with small LV size and marked hypertrophy.157162 This scenario may also result from conditions associated with low stroke volume (e.g. moderate/severe mitral regurgitation, severe tricuspid regurgitation, severe mitral stenosis, and large ventricular septal defect and severe RV dysfunction). Diagnosis of severe aortic stenosis is challenging and requires careful exclusion of measurement errors and other explanations for the echocardiographic findings,25 as well as the presence or absence of typical symptoms (with no other explanation), LV hypertrophy (in the absence of coexistent hypertension) or reduced LV longitudinal strain (with no other cause). CCT assessment of the degree of valve calcification provides important additional information [thresholds (Agatston units) for severe aortic stenosis: men >3000, women >1600 = highly likely; men >2000, women >1200 = likely; men <1600, women <800 = unlikely].3536163164
- Normal-flow, low-gradient aortic stenosis with preserved ejection fraction (mean gradient <40 mmHg, valve area ≤1 cm2, LVEF≥50%, SVi >35 mL/m2). These patients usually have only moderate aortic stenosis.36165166167
5.1.2 ADDITIONAL DIAGNOSTIC AND PROGNOSTIC PARAMETERS
The resting Doppler velocity index (DVI, also termed ‘dimensionless index’) –the ratio of the left ventricular outflow tract (LVOT) time-velocity integral (TVI) to that of the aortic valve jet– does not require calculation of LVOT area and may assist evaluation when other parameters are equivocal (a value <0.25 suggests that severe aortic stenosis is highly likely).156 Assessment of global longitudinal strain provides additional information concerning LV function and a threshold of 15% may help to identify patients with severe asymptomatic aortic stenosis who are at higher risk of clinical deterioration or premature mortality.26168 TOE allows evaluation of concomitant mitral valve disease and may be of value for periprocedural imaging during TAVI and SAVR.169
Natriuretic peptides predict symptom-free survival and outcome in normal and low-flow severe aortic stenosis.170171 They can be used to arbitrate the source of symptoms in patients with multiple potential causes and identify those with high-risk asymptomatic aortic stenosis who may benefit from early intervention (section 5.2.2, Table 6 and Figure 3).
Exercise testing may unmask symptoms and is recommended for risk stratification of asymptomatic patients with severe aortic stenosis.172 Exercise echocardiography provides additional prognostic information by assessing the increase in mean pressure gradient and change in LV function.173
CCT provides information concerning the anatomy of the aortic root and ascending aorta, and the extent and distribution of valve and vascular calcification, and feasibility of vascular access.174 Quantification of valve calcification predicts disease progression and clinical events164 and may be useful when combined with geometric assessment of valve area in assessing the severity of aortic stenosis in patients with low valve gradient.3536163164
Myocardial fibrosis is a major driver of LV decompensation in aortic stenosis (regardless of the presence or absence of CAD), which can be detected and quantified using CMR. Amyloidosis is also frequently associated with aortic stenosis in elderly patients (incidence 9-15%).175 When cardiac amyloidosis is clinically suspected, based on symptoms (neuropathy and hematologic data), diphosphonate scintigraphy and/or CMR should be considered. Both entities persist following valve intervention and are associated with poor long-term prognosis.176177178179
Coronary angiography is essential prior to TAVI and SAVR to determine the potential need for concomitant revascularization (see section 3.2.4.1 and section 5.5). Retrograde LV catheterization is not recommended unless there are symptoms and signs of severe aortic stenosis and non-invasive investigations are inconclusive.
5.1.3 TAVI DIAGNOSTIC WORKUP
Prior to TAVI, CCT is the preferred imaging tool to assess: (i) aortic valve anatomy, (ii) annular size and shape, (iii) extent and distribution of valve and vascular calcification, (iv) risk of coronary ostial obstruction, (v) aortic root dimensions, (vi) optimal fluoroscopic projections for valve deployment, and (vii) feasibility of vascular access (femoral, subclavian, axillary, carotid, transcaval or transapical). Adverse anatomical findings may suggest that SAVR is a better treatment option (Table 6). TOE is more operator-dependent but may be considered when CCT is difficult to interpret or relatively contraindicated (e.g. chronic renal failure).
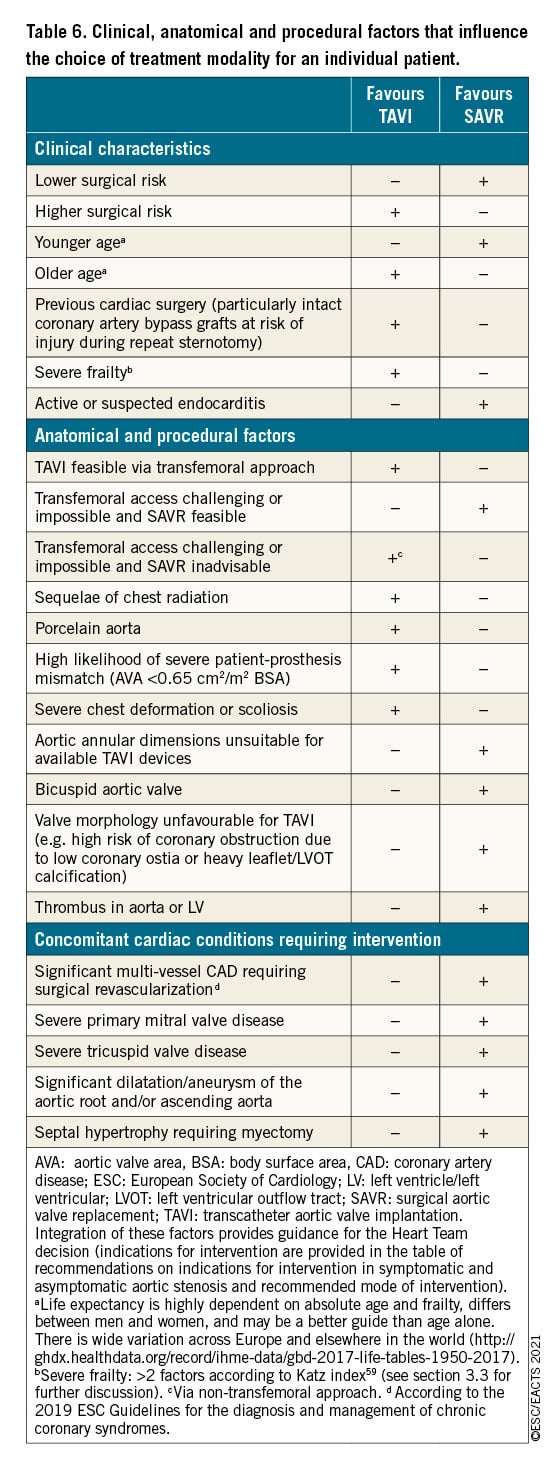
5.2 INDICATIONS FOR INTERVENTION (SAVR OR TAVI)
Indications for aortic valve intervention are summarized in the table of recommendations on indications for intervention in symptomatic and asymptomatic aortic stenosis and recommended mode of intervention and in Figure 4.
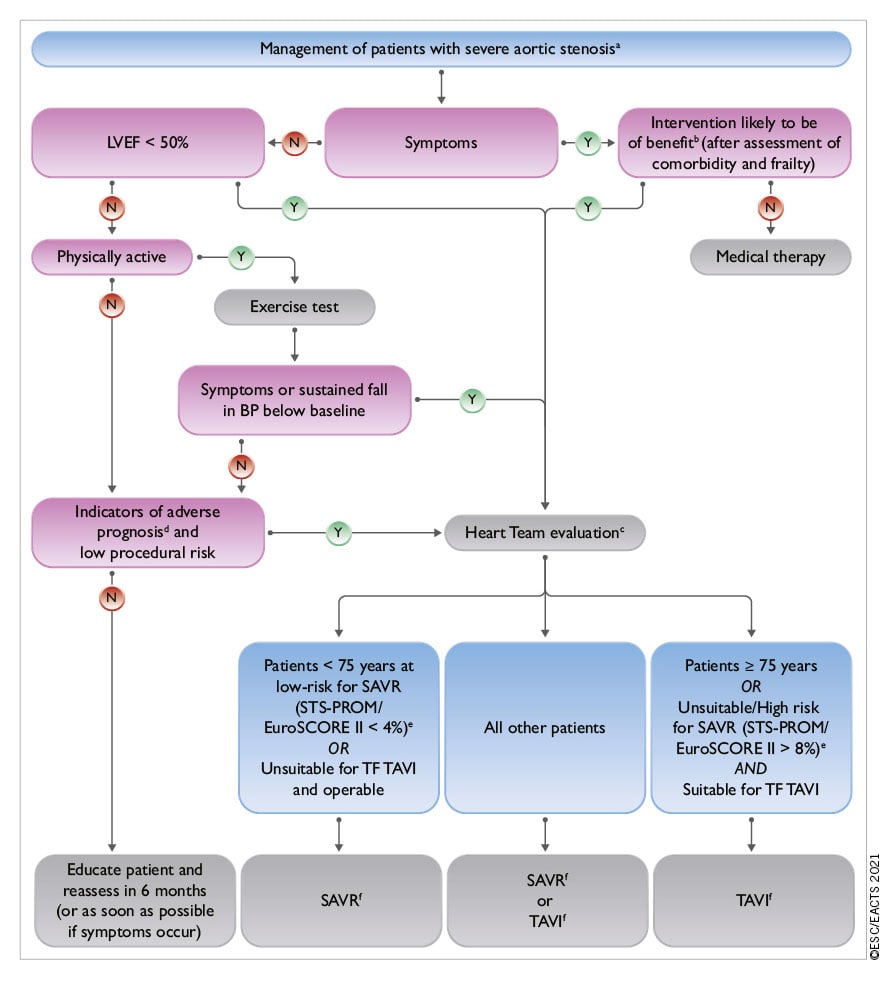
Figure 4. Management of patients with severe aortic stenosis. BP: blood pressure; EuroSCORE: European System for Cardiac Operative Risk Evaluation; LVEF: left ventricular ejection fraction; SAVR: surgical aortic valve replacement; STS-PROM: Society of Thoracic Surgeons – predicted risk of mortality; TAVI: transcatheter aortic valve implantation; TF: transfemoral. aSee Figure 3: Integrated imaging assessment of aortic stenosis. bProhibitive risk is defined in Supplementary Table 5. cHeart Team assessment based upon careful evaluation of clinical, anatomical, and procedural factors (see Table 6 and table on Recommendations on indications for intervention in symptomatic and asymptomatic aortic stenosis and recommended mode of intervention). The Heart Team recommendation should be discussed with the patient who can then make an informed treatment choice. dAdverse features according to clinical, imaging (echocardiography/CT), and/or biomarker assessment. eSTS-PROM: http://riskcalc.sts.org/stswebriskcalc/#/calculate, EuroSCORE II: http://www.euroscore.org/calc.html. fIf suitable for procedure according to clinical, anatomical, and procedural factors (Table 6).
5.2.1 SYMPTOMATIC AORTIC STENOSIS
Symptomatic severe aortic stenosis has dismal prognosis and early intervention is strongly recommended in all patients. The only exceptions are for those in whom intervention is unlikely to improve quality of life or survival (due to severe comorbidities) or for those with concomitant conditions associated with survival <1 year (e.g. malignancy) (section 3).
Intervention is recommended in symptomatic patients with high-gradient aortic stenosis, regardless of LVEF. However, management of patients with low-gradient aortic stenosis is more challenging:
- LV function usually improves after intervention in patients with low-flow, low-gradient aortic stenosis, when reduced ejection fraction is predominantly caused by excessive afterload.32180 Conversely, improvement is uncertain if the primary cause of reduced ejection fraction is scarring due to myocardial infarction or cardiomyopathy. Intervention is recommended when severe aortic stenosis is confirmed by stress echocardiography (true severe aortic stenosis; Figure 3),32 while patients with pseudo-severe aortic stenosis should receive conventional heart failure treatment.142181 The presence or absence of flow reserve (increase in stroke volume ≥20%) d [oe]s not appear to influence prognosis in contemporary series of patients undergoing TAVI or SAVR,182183184 and although those with no flow reserve show increased procedural mortality, both modes of intervention improve ejection fraction and clinical outcomes.32180182 Decision making for such patients should take account of comorbidities, degree of valve calcification, extent of CAD, and feasibility of revascularization.
- Data concerning the natural history of low-flow, low-gradient aortic stenosis and preserved ejection fraction, and outcomes after SAVR and TAVI remain controversial.162165167 Intervention should only be considered in those with symptoms and significant valve obstruction (see table of recommendations on indications for intervention in symptomatic and asymptomatic aortic stenosis and recommended mode of intervention and Figure 4).
- The prognosis of patients with normal-flow, low-gradient aortic stenosis and preserved ejection fraction is similar to that of moderate aortic stenosis –regular clinical and echocardiographic surveillance is recommended.165166185
5.2.2 ASYMPTOMATIC AORTIC STENOSIS
Intervention is recommended in asymptomatic patients with severe aortic stenosis and impaired LV function of no other cause,9 and those who are asymptomatic during normal activities but develop symptoms during exercise testing.172186 Management of asymptomatic severe aortic stenosis is otherwise controversial and the decision to intervene requires careful assessment of the benefits and risks in an individual patient.
In the absence of adverse prognostic features, watchful waiting has generally been recommended with prompt intervention at symptom onset.187 Data from a single RCT have shown significant reduction in the primary endpoint (death during or within 30 days of surgery or cardiovascular death during the entire follow-up period) following early SAVR compared with conservative management [1% vs. 15%; hazard ratio 0.09; 95% confidence interval (CI), 0.01-0.67; P = 0.003].188 However, subjects were selected per inclusion criteria (median age 64 years, minimal comorbidities, low operative risk) and follow-up in the conservative group was limited. Further randomized trials [EARLY TAVR (NCT03042104), AVATAR (NCT02436655), EASY-AS (NCT04204915), EVOLVED (NCT03094143)] will help determine future recommendations.
Predictors of symptom development and adverse outcomes in asymptomatic patients include clinical characteristics (older age, atherosclerotic risk factors), echocardiographic parameters (valve calcification, peak jet velocity189190), LVEF, rate of haemodynamic progression,189 increase in mean gradient >20 mmHg with exercise,172 severe LV hypertrophy,191 indexed stroke volume,158 LA volume,192 LV global longitudinal strain,26168193 and abnormal biomarker levels (natriuretic peptides, troponin, and fetuin-A).170171194195 Early intervention may be considered in asymptomatic patients with severe aortic stenosis and one or more of these predictors if procedural risk is low (although application of TAVI in this setting has yet to be formally evaluated) (Table 6 and Figure 4). Otherwise, watchful waiting is a safer and more appropriate strategy.
5.2.3 THE MODE OF INTERVENTION
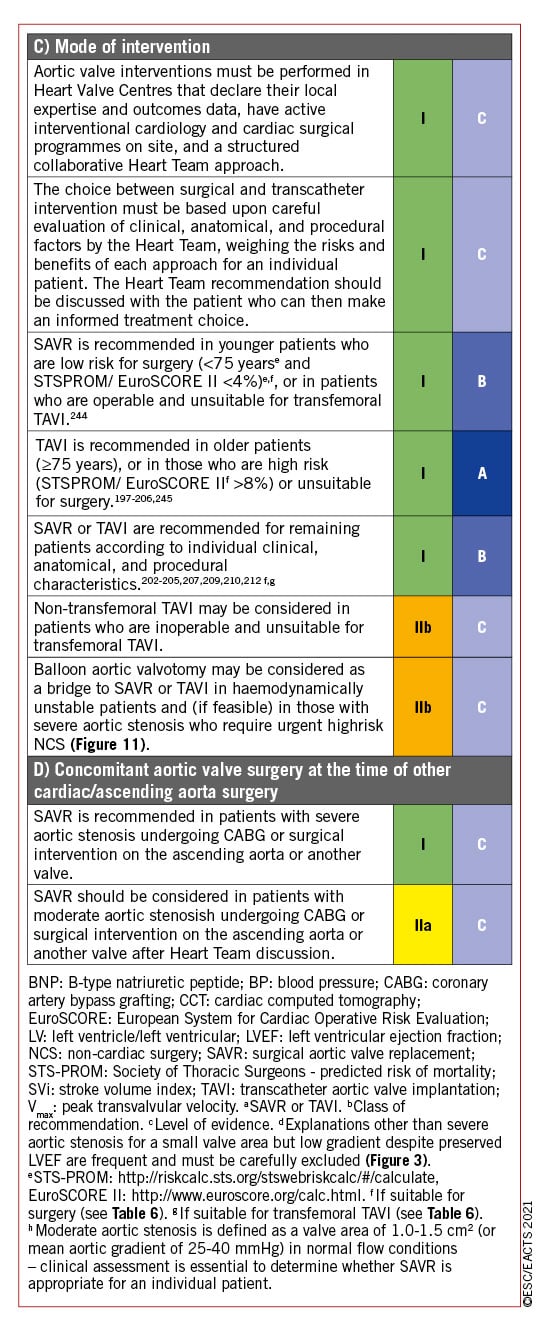
Use of SAVR and TAVI as complementary treatment options has allowed a substantial increase in the overall number of patients with aortic stenosis undergoing surgical or transcatheter intervention in the past decade.196 RCTs have assessed the two modes of intervention across the spectrum of surgical risk in predominantly elderly patients and a detailed appraisal of the evidence base is provided in Supplementary Section 5. In brief, these trials used surgical risk scores to govern patient selection and demonstrate that TAVI is superior to medical therapy in extreme-risk patients,197 and non-inferior to SAVR in high-198199200201 and intermediate-risk patients at follow-up extending to 5 years.202203204205206207208 The more recent PARTNER 3 and Evolut Low Risk trials demonstrate that TAVI is non-inferior to SAVR in low-risk patients at 2-year follow-up.209210211212 Importantly, patients in the low-risk trials were predominantly male and relatively elderly (e.g. PARTNER 3: mean age 73.4 years, <70 years 24%, 70-75 years 36%, >75 years 40%, >80 years 13%) whilst those with low-flow aortic stenosis or adverse anatomical characteristics for either procedure (including bicuspid aortic valves or complex coronary disease) were excluded.
Rates of vascular complications, pacemaker implantation, and paravalvular regurgitation are consistently higher after TAVI, whereas severe bleeding, acute kidney injury, and new-onset AF are more frequent after SAVR. Although the likelihood of paravalvular regurgitation has been reduced with newer transcatheter heart valve designs, pacemaker implantation (and new-onset left bundle branch block) may have long-term consequences213214215 and further refinements are required. Most patients undergoing TAVI have a swift recovery, short hospital stay, and rapidly return to normal activities.216217 Despite these benefits, there is wide variation in worldwide access to the procedure as a result of high device costs and differing levels of healthcare resources.71218219
The Task Force has attempted to address the gaps in evidence and provide recommendations concerning the indications for intervention and mode of treatment (Recommendations on indications for intervention in symptomatic and asymptomatic aortic stenosis and recommended mode of intervention, Figure 4) that are guided by the RCT findings and compatible with real-world Heart Team decision making for individual patients (many of whom fall outside the RCT inclusion criteria). Aortic stenosis is a heterogeneous condition and selection of the most appropriate mode of intervention should be carefully considered by the Heart Team for all patients, accounting for individual age and estimated life expectancy, comorbidities (including frailty and overall quality of life, section 3), anatomical and procedural characteristics (Table 6), the relative risks of SAVR and TAVI and their long-term outcomes, prosthetic heart valve durability, feasibility of transfemoral TAVI, and local experience and outcome data. These factors should be discussed with the patient and their family to allow informed treatment choice.
The interplay between estimated life expectancy and prosthetic heart valve durability is a key consideration in these discussions. Age is a surrogate for life expectancy but had no impact on the outcomes of the low-risk RCTs at 1-2 year follow-up. Life expectancy varies widely across the world and is highly dependent on absolute age, sex, frailty, and the presence of comorbidities (http://ghdx.healthdata.org/record/ihme-data/gbd-2017-life-tables-1950-2017); it may be a better guide than age alone but is difficult to determine in individual patients. Although some (now abandoned) surgical bioprosthetic designs have failed early, the durability of contemporary surgical bioprosthetic valves beyond 10 years is well established.220 Conversely, registry data provide some reassurance concerning the long-term durability of TAVI devices up to 8 years but largely relate to older high-/intermediate-risk patients,221222223224 whereas information concerning durability in low-risk patients is currently limited to 2-year follow-up. Data comparing the durability of transcatheter heart valves and surgical bioprostheses directly remain limited. Rates of aortic valve re-intervention were higher after TAVI using a balloon-expandable valve compared to SAVR at 5-year follow-up in the PARTNER 2A trial (3.2% vs. 0.8%; hazard ratio, 3.3; 95% CI, 1.3-8.1),206 whereas rates of structural valve deterioration (SVD) were not statistically different following SAVR and TAVI using the third generation SAPIEN 3 device in a parallel observational registry over the same time frame.225
Valve-in-valve TAVI is an established treatment option for surgical bioprosthetic valve deterioration but may not be appropriate or feasible in all patients due to the increased likelihood of PPM in patients with a small aortic root (or undersized original prosthesis), incompatible surgical valve designs associated with increased risk of coronary occlusion, or difficult vascular access; re-do SAVR should also be considered in these settings.226227228 Favourable short-term outcomes of redo-TAVI have been demonstrated in selected older patients with transcatheter heart valve deterioration,229 despite theoretical concerns relating to maintained coronary access.230
A bicuspid aortic valve is more frequent in younger patients with aortic stenosis. While several registries have reported excellent outcomes of TAVI in patients with a bicuspid valve who were unsuitable for surgery,231232233 SAVR remains more appropriate in patients with aortic stenosis affecting a bicuspid valve and in those with associated disease (e.g. aortic root dilatation, complex coronary disease, or severe mitral regurgitation) requiring a surgical approach.
In summary, prosthetic heart valve durability is a key consideration in younger patients (<75 years) at low surgical risk and SAVR (if feasible) is therefore the preferred treatment option. Conversely, durability is a lower priority in older patients (≥75 years), or those who are inoperable or high risk for surgery, and TAVI is preferred in these groups (particularly if feasible via transfemoral approach). The Heart Team should make tailored recommendations for remaining patients based upon their individual characteristics (Table 6). This guidance should be re-addressed when further data concerning the long-term durability of TAVI become available.
Balloon aortic valvuloplasty (BAV) may be considered as a bridge to TAVI or SAVR in patients with decompensated aortic stenosis and (when feasible) in those with severe aortic stenosis who require urgent high-risk non-cardiac surgery (NCS) (section 12). The procedure carries significant risk of complications234 and should only be undertaken after Heart Team discussion.
5.3 MEDICAL THERAPY
No medical therapies influence the natural history of aortic stenosis. Statins (which demonstrated favourable effects in pre-clinical studies) do not affect disease progression246 and clinical trials targeting calcium metabolic pathways are ongoing. Patients with heart failure who are unsuitable (or waiting) for SAVR or TAVI should be medically treated according to ESC heart failure Guidelines.247 ACEI are safe in aortic stenosis (provided that BP is monitored carefully) and may have beneficial myocardial effects before the onset of symptoms, and after TAVI and SAVR.248249250 Coexisting hypertension should be treated to avoid additional afterload, although medication (particularly vasodilators) should be titrated to avoid symptomatic hypotension.
Antithrombotic therapy after TAVI is discussed in section 11.
5.4 SERIAL TESTING
Rate of progression of aortic stenosis varies widely. Asymptomatic patients, their family and medical carers need careful education, with emphasis on the importance of regular follow-up (ideally in a Heart Valve Clinic9) and prompt reporting of symptoms. Those with severe aortic stenosis should be followed up every 6 months (at least) to allow earliest symptom detection (using exercise testing if symptoms are doubtful) and any change in echocardiographic parameters (particularly LVEF). Measurement of natriuretic peptides may be considered.
Several studies suggest that the prognosis of moderate degenerative aortic stenosis is worse than previously considered251252253254 (particularly if there is significant valve calcification) and these patients should be re-evaluated at least annually. Younger patients with mild aortic stenosis and no significant calcification may be followed up every 2-3 years.
5.5 SPECIAL PATIENT POPULATIONS
Women with aortic stenosis have higher mortality than men, resulting from late diagnosis and initial specialist assessment followed by less frequent and delayed referral for intervention.255256257 Measures are needed to improve this situation and ensure that both sexes receive equivalent care.
CAD and aortic stenosis frequently coexist and the combination confers higher risk of clinical events, therefore the need to consider revascularization in conjunction with aortic valve intervention is common. The impact of coronary revascularization in patients with silent CAD accompanying aortic stenosis is unclear and further studies are warranted in this context (section 3). Both simultaneous SAVR and CABG, and SAVR late after CABG, carry a higher procedural risk than isolated SAVR. Nevertheless, retrospective data indicate that patients with moderate aortic stenosis, in whom CABG is indicated, benefit from concomitant SAVR. Patients aged <70 years with mean gradient progression ≥5 mmHg/year benefit from SAVR at the time of CABG once baseline peak gradient exceeds 30 mmHg.258 Decisions for individual patients should take into account haemodynamic data, rate of progression, extent of leaflet calcification, life expectancy, and associated comorbidities, as well as the individual risk of concomitant SAVR or deferred TAVI.244
Percutaneous coronary intervention (PCI) and TAVI may be undertaken as combined or staged procedures according to the clinical situation, pattern of CAD, and extent of myocardium at risk.259 In the SURTAVI trial, there was no significant difference in the primary endpoint (all-cause mortality or stroke at 2-year follow-up) in intermediate-risk patients with severe aortic stenosis and non-complex CAD (SYNTAX score <22) undergoing either TAVI and PCI or SAVR and CABG [16.0% (95% CI, 11.1-22.9) vs. 14% (95% CI, 9.2-21.1); P = 0.62].260 Assessing the clinical value of systematic PCI in TAVI patients with significant associated CAD is the objective of ongoing RCTs. Patients with severe symptomatic aortic stenosis and diffuse CAD unsuitable for revascularization should receive optimal medical therapy and undergo SAVR or TAVI according to individual characteristics.
Severity of mitral regurgitation accompanying severe aortic stenosis may be overestimated as a result of elevated LV pressures and careful quantification is required. In patients with severe primary mitral regurgitation (PMR), mitral valve surgery is required at the time of SAVR. In patients with severe SMR, surgery may also be considered in the presence of significant annular dilatation and marked LV enlargement. In high-risk or inoperable patients with severe aortic stenosis and severe mitral regurgitation, combined (or more often sequential) TAVI and TEER may be feasible, but there is insufficient experience to allow robust recommendations.261262263 In patients with severe PMR, TEER should be considered early if the patient remains symptomatic and mitral regurgitation is still severe after TAVI. In patients with severe SMR, TAVI should be followed by careful clinical and echocardiographic reassessment to determine whether further mitral intervention is required.264
Section 4 provides recommendations for the management of aneurysm/dilatation of the ascending aorta accompanying aortic stenosis. Assessment and management of congenital aortic stenosis is addressed in ESC Guidelines on adult congenital heart disease.265
6 Mitral regurgitation
Mitral regurgitation is the second-most frequent VHD in Europe.13 The underlying mechanism (primary or secondary) determines the therapeutic approach.
6.1 PRIMARY MITRAL REGURGITATION
Primary lesion of one or more components of the mitral valve apparatus characterizes PMR. Degenerative aetiology (fibroelastic deficiency and Barlow disease) is most frequent in Western countries.12266 In low-income countries, rheumatic aetiology is the most frequent cause of mitral regurgitation.267 Endocarditis can cause PMR and is addressed in the corresponding ESC Guidelines.4
6.1.1 EVALUATION
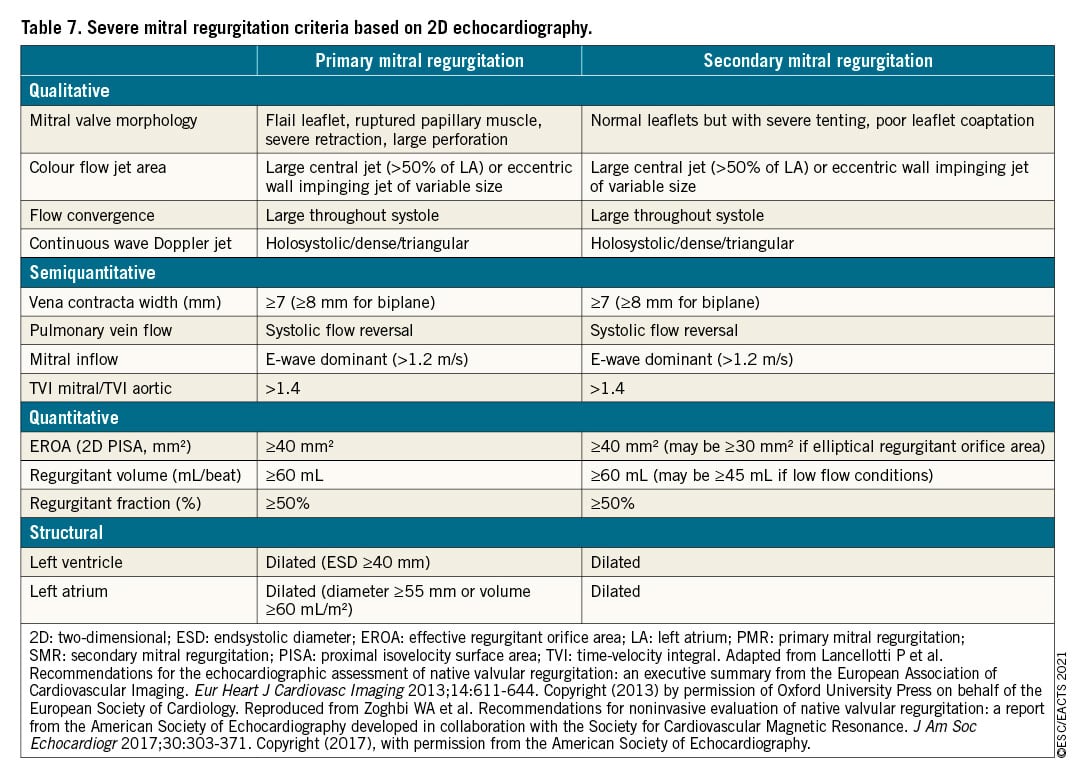
Echocardiography is the first choice of imaging technique to grade PMR (Table 7). An integrative approach including qualitative, semi-quantitative, and quantitative measures of mitral regurgitation (besides quantification of LV and LA dimensions) is recommended.24268 Routinely measured effective regurgitant orifice area (EROA) is strongly associated with all-cause mortality, and compared with the general population an excess mortality appears for an EROA ≥20 mm2 and steadily increases beyond 40 mm2.269 Evaluation of the specific lesion leading to mitral regurgitation has prognostic implications266270 and is fundamental to determine the feasibility of surgical and transcatheter valve repair271272273 (Supplementary Figure 1). Three-dimensional TOE provides an ‘en face’ view of the mitral leaflets resembling the surgical inspection of the valve, thereby facilitating Heart Team discussion.24268 In addition, 3D echocardiography has shown better agreement with CMR in quantifying the regurgitant volume than 2D echocardiography, particularly in eccentric, multiple and late-systolic regurgitant jets.274275276277 When various echocardiographic parameters used to grade mitral regurgitation are inconsistent, CMR is a valid alternative to quantify the regurgitant volume and is the reference standard to quantify LV and LA volumes.278 In addition, quantification of mitral regurgitation with CMR has shown prognostic implications.277 Finally, preliminary data show that myocardial fibrosis assessed with CMR is frequent in PMR and has been associated with sudden cardiac death and ventricular arrhythmias.279
Exercise echocardiography permits evaluation of changes in mitral regurgitant volume and pulmonary pressures during peak exercise and is particularly helpful in patients with discordant symptoms and regurgitation grade at rest.280281 In asymptomatic patients with severe PMR and non-dilated LV and LA, low BNP values are associated with low mortality and can be useful during follow-up.41282
LV dimensions and ejection fraction are considered to guide the management of patients with severe PMR. However, there is cumulative evidence showing that LV global longitudinal strain has incremental prognostic value in patients treated with surgical repair.283284 Recently, the Mitral Regurgitation International Database (MIDA) score has been proposed to estimate the risk of all-cause mortality in patients with severe PMR due to flail leaflet, who are under medical treatment or surgically treated.285 Among the variables included in the score, LA diameter ≥55 mm and LVESD ≥40 mm are new thresholds that have been included in the current recommendations.
Right heart catheterization is systematically used to confirm pulmonary hypertension diagnosed by echocardiography when this is the only criterion to refer the patient for surgery.
6.1.2 INDICATIONS FOR INTERVENTION
Urgent surgery is indicated in patients with acute severe mitral regurgitation. In the case of papillary muscle rupture as the underlying disease, valve replacement is generally required.
Indications for surgery in severe chronic PMR are shown in the following table of recommendations and in Figure 5. Surgery is recommended in patients with symptomatic severe PMR and acceptable surgical risk according to the Heart Team decision. The presence of LVEF ≤60%, LVESD ≥40 mm,285286 LA volume ≥60 mL/m2 or diameter ≥55 mm,287288 systolic pulmonary arterial pressure (SPAP) >50 mmHg,289 and AF290291 have been associated with worse outcomes and are considered triggers for intervention regardless of symptomatic status.292 In the absence of these criteria, watchful waiting is a safe strategy in asymptomatic patients with severe PMR and ideally should be performed in a Heart Valve Clinic.
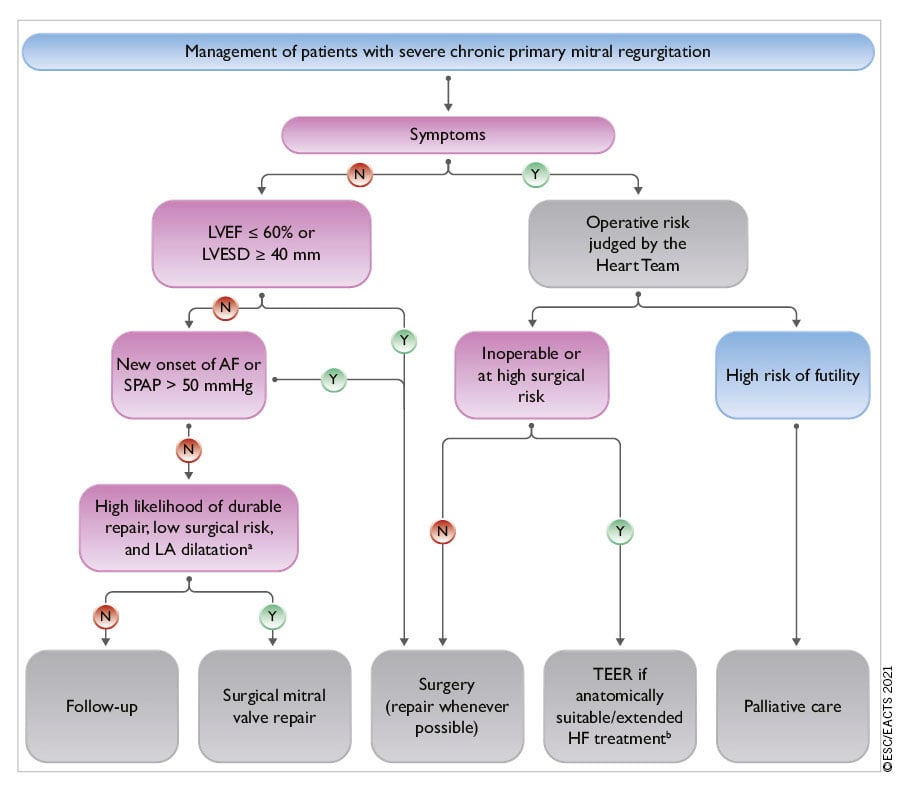
Figure 5. Management of patients with severe chronic primary mitral regurgitation. AF: atrial fibrillation; HF: heart failure; LA: left atrium/ left atrial; LVEF: left ventricular ejection fraction; LVESD: left ventricular end-systolic diameter; SPAP: systolic pulmonary arterial pressure; TEER: transcatheter edge-to-edge repair. aLA dilatation: volume index ≥60 mL/m2 or diameter ≥55 mm at sinus rhythm. bExtended heart failure treatment includes the following: CRT; ventricular assist devices; heart transplantation.247
When surgery is considered, mitral valve repair is the surgical intervention of first choice when the results are expected to be durable according to the Heart Team evaluation since it is associated with better survival compared to mitral valve replacement.293294 PMR due to segmental valve prolapse can be repaired with a low risk of recurrence and reoperation.294295296 The reparability of rheumatic lesions, extensive valve prolapse and to a greater extent leaflet calcification or extensive annular calcification is more challenging.297298 Patients requiring a predictably complex repair should undergo surgery in experienced repair centres with high repair rates, low operative mortality, and a record of durable results. When repair is not feasible, mitral valve replacement with preservation of the subvalvular apparatus is favoured.
Transcatheter mitral valve implantation for severe PMR is a safe alternative in patients with contraindications for surgery or high operative risk.299300301302 TEER is the most evidenced, while the safety and efficacy of other techniques have been demonstrated in smaller series.303304305306 The efficacy of more recent TEER system iterations307 will be investigated in high-risk (MITRA-HR study NCT03271762)308 and intermediate-risk patients (REPAIR-MR study NCT04198870).

6.1.3 MEDICAL THERAPY
In acute mitral regurgitation, nitrates and diuretics are used to reduce filling pressures. Sodium nitroprusside reduces afterload and regurgitant fraction. Inotropic agents and an intra-aortic balloon pump are of use in hypotension and haemodynamic instability.
In chronic PMR with preserved LVEF, there is no evidence to support the prophylactic use of vasodilators. In patients with overt heart failure, medical treatment as per current heart failure guidelines applies.247
6.1.4 SERIAL TESTING
Asymptomatic patients with severe mitral regurgitation and LVEF >60% should be followed clinically and by echocardiography every 6 months, ideally in the setting of a Heart Valve Centre.309 Measurement of BNP levels, exercise echocardiography, electrocardiogram-Holter monitoring and CMR are useful complementary diagnostic and risk stratification tools.268 The association between PMR, sudden cardiac death and ventricular arrhythmias remains controversial.310311312 The presence of mitral annulus disjunction (abnormal atrial displacement of the hinge point of the mitral valve away from the ventricular myocardium) has been also associated with increased risk of ventricular arrhythmias.310311313 Interestingly, the majority of these patients did not have severe mitral regurgitation. In asymptomatic patients with severe PMR and progressive increase of LV size (LVESD approaching 40 mm) or decrease of LVEF on serial studies, surgical mitral valve repair should be discussed. Asymptomatic patients with moderate mitral regurgitation and preserved LV function can be followed on a yearly basis and echocardiography should be performed every 1-2 years. After intervention, serial follow-up focuses on evaluation of symptomatic status, presence of arrhythmic events, assessment of valve function,314 and recurrence of mitral regurgitation. After surgical mitral valve repair, high-volume centres have reported good durability with a recurrence rate of moderate or severe mitral regurgitation of 12.5% at 20 years of follow-up.296 After transcatheter mitral valve repair, the currently reported rates of residual moderate and severe mitral regurgitation (23-30%) would suggest that yearly echocardiogram is appropriate.14300301
6.1.5 SPECIAL POPULATIONS
Sex differences in terms of prevalence of underlying aetiology of PMR and management have been reported.298315316 Despite the reduction in the prevalence of rheumatic disease in Western countries, women still have higher rates of rheumatic mitral regurgitation than men and emerging aetiologies such as radiation heart disease are also more frequent in women.297 These aetiologies are often characterized by severe calcification of the mitral valve apparatus and associated with mitral stenosis precluding durable repair. Women with PMR referred for surgical treatment received mitral valve repair at a similar rate to men.316 However, women more frequently present with post-operative heart failure, probably related to a later referral and more advanced disease as compared to men.
6.2 SECONDARY MITRAL REGURGITATION
In SMR, the valve leaflets and chordae are structurally normal and mitral regurgitation results from an imbalance between closing and tethering forces secondary to alterations in LV and LA geometry.317318 It is most commonly seen in dilated or ischaemic cardiomyopathies, both in severely dilated LV with markedly depressed LV function or after an isolated infero-basal myocardial infarction leading to posterior leaflet tethering, despite almost normal LV size and ejection fraction. SMR may also arise as a consequence of LA enlargement and mitral annular dilatation in patients with longstanding AF, in whom LVEF is usually normal and LV dilatation less pronounced (so called ‘atrial functional mitral regurgitation’).319
6.2.1 EVALUATION
The echocardiographic criteria to define severe SMR do not differ from those used in PMR and an integrative approach should be used (Table 7).24268 However, it should be acknowledged that when quantifying EROA and regurgitant volume in SMR, lower thresholds may be applied to define severe SMR. In heart failure patients, the total forward LV stroke volume is lower and that may lead to lower estimated regurgitant volume (<60 mL/beat). Calculation of regurgitant fraction in those circumstances could account for lower flows and has shown prognostic implications.320 In addition, the crescent shape of the regurgitant orifice, characteristic of SMR, may lead to underestimation of the vena contracta width and of the EROA. An EROA ≥30 mm2 by 2D proximal isovelocity surface area (PISA) likely corresponds to severe SMR. In contrast, whether an EROA ≥20 mm2 defines severe SMR remains controversial. In heart failure patients, even mild mitral regurgitation is associated with poor prognosis321 and evidence that surgical or transcatheter treatment of moderate SMR does not seem to improve patient outcomes322323 supports the change in definition of severe SMR. Caution is required, therefore, when labelling severe SMR based solely on prognostic implications. Other factors such as the extent of myocardial scar, as assessed with CMR, have been associated with poor prognosis.324 In addition, LVEF has been shown to be misleading in patients with severe SMR, while LV global longitudinal strain has been shown to have incremental prognostic value.325326 The use of 3D echocardiography, CMR and exercise echocardiography may help to identify patients with severe mitral regurgitation when 2D echocardiography at rest is inconclusive.24268
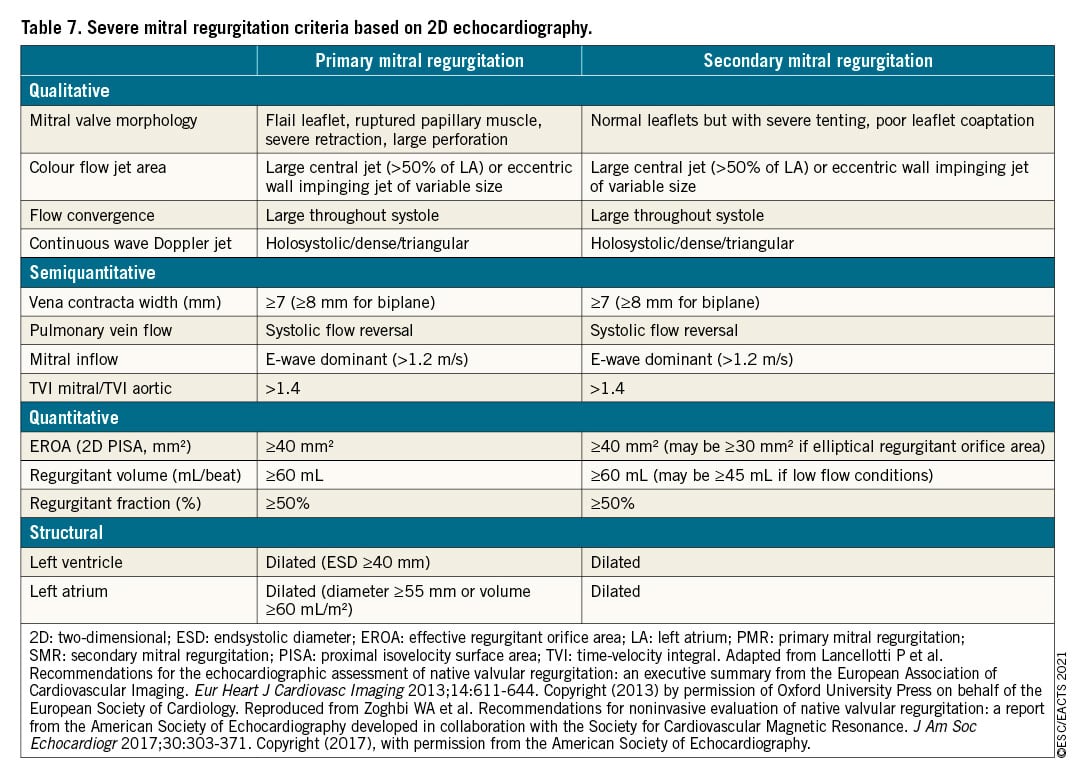
6.2.2 MEDICAL THERAPY
Optimal medical therapy in line with the guidelines for the management of heart failure247 should be the first and essential step in the management of all patients with SMR and should include replacement of ACEI or ARB with sacubitril/valsartan, sodium-glucose co-transporter 2 inhibitors and/or ivabradine, whenever indicated.247327 Indications for cardiac resynchronization therapy (CRT) should be evaluated in accordance with related guidelines.247 If symptoms persist after optimization of conventional heart failure therapy, options for mitral valve intervention should be promptly evaluated before further deterioration of LV systolic function or cardiac remodelling occur.
6.2.3 INDICATIONS FOR INTERVENTION
Chronic SMR is associated with impaired prognosis321328 and its interventional management is complex (see recommendations on indications for mitral valve intervention in chronic severe SMR, and Figure 6). The detailed analysis of the available level of evidence made by the methodology group of the task force is available in Supplementary Section 5. The importance of decision making by a multidisciplinary Heart Team needs to be emphasized in this setting. The Heart Team, including a heart failure specialist, should optimize guideline-directed medical therapy (GDMT) and consider the indications of electrophysiological, transcatheter and surgical interventions, their priority and order of implementation.
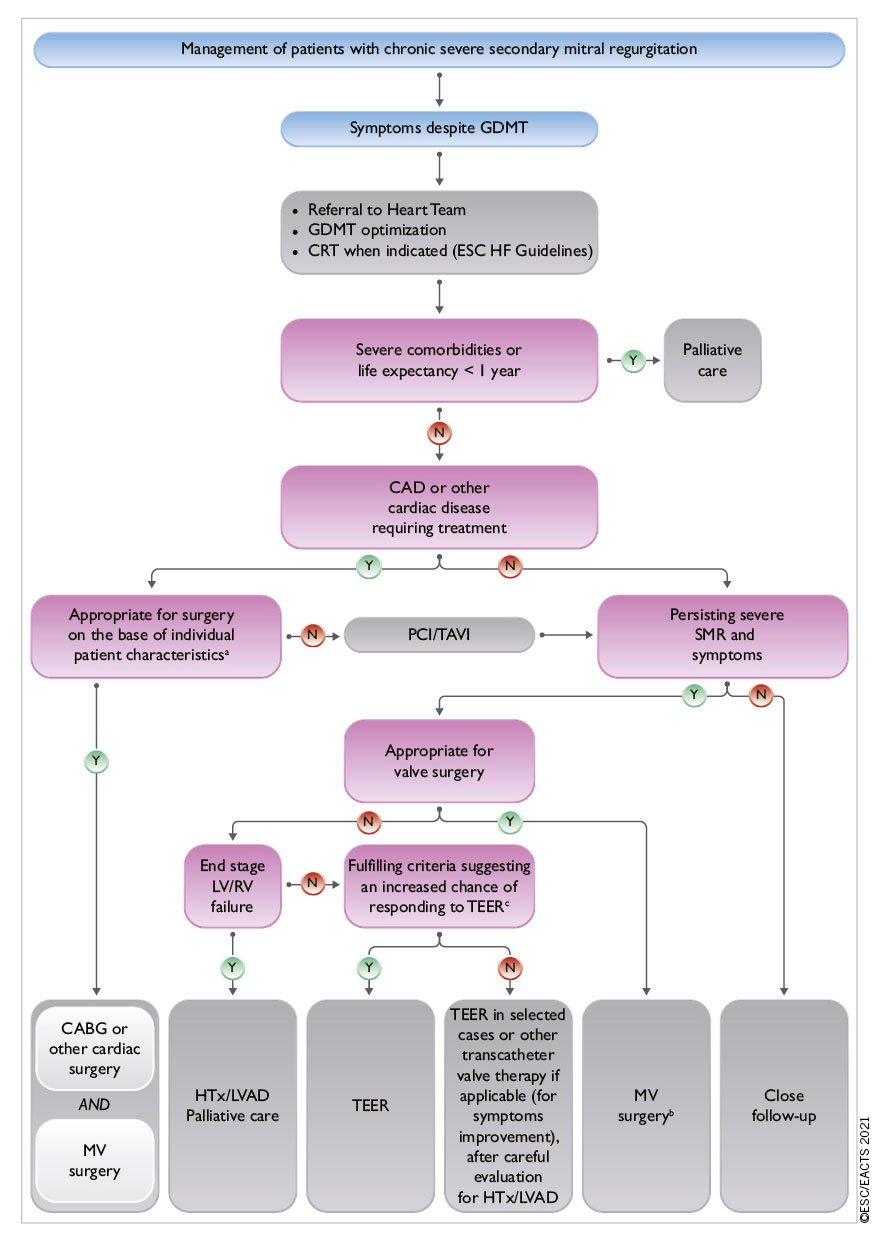
Figure 6. Management of patients with chronic severe secondary mitral regurgitation. CAD: coronary artery disease; CABG: coronary artery bypass grafting; CRT: cardiac resynchronization therapy; ESC: European Society of Cardiology; GDMT: guideline-directed medical therapy; HF: heart failure; HTx: heart transplantation; LVAD: left ventricular assist devices; LV: left ventricle/left ventricular; LVEF: left ventricular ejection fraction; MV: mitral valve; PCI: percutaneous coronary intervention; RV: right ventricle/right ventricular; SMR: secondary mitral regurgitation; TAVI: transcatheter aortic valve implantation; TEER: transcatheter edge-to-edge repair. aLVEF, predicted surgical risk, amount of myocardial viability, coronary anatomy/target vessels, type of concomitant procedure needed, TEER eligibility, likelihood of durable surgical repair, need of surgical mitral replacement, local expertise. bParticularly when concomitant tricuspid valve surgery is needed. cCOAPT criteria (Cardiovascular Outcomes Assessment of the MitraClip Percutaneous Therapy for Heart Failure Patients With Functional Mitral Regurgitation): see Supplementary Table 7.
The evidence supporting surgical intervention remains limited. Mitral valve surgery is recommended in patients with severe SMR undergoing CABG or other cardiac surgery.329330 The surgical approach has to be tailored to the individual patient.247331 In selected patients without advanced LV remodelling, mitral valve repair with an undersized complete rigid ring restores valve competence, improves symptoms, and results in reverse LV remodelling.331 Additional valvular/subvalvular techniques or chordal sparing valve replacement may be considered in patients with echocardiographic predictors of repair failure.332 Valve replacement avoids recurrence of mitral regurgitation, although this does not translate into better LV reverse remodelling or survival.333 Indications for isolated mitral valve surgery in SMR are particularly restrictive, owing to significant procedural risk, high rates of recurrent mitral regurgitation, and the absence of proven survival benefit.333334335 In patients with atrial functional mitral regurgitation, LVEF is usually normal, LV dilatation less pronounced and mitral annular dilatation represents the main mechanism of mitral regurgitation. This subgroup may be more effectively treated by ring annuloplasty often associated with ablation of AF but evidence is still limited.319
TEER with the MitraClip system is a minimal-invasive treatment option for SMR. Two RCTs (COAPT and MITRA-FR)323336337 have evaluated its safety and efficacy in patients with symptomatic heart failure and severe SMR persisting despite medical therapy, who were considered either ineligible or not appropriate for surgery by the Heart Team (Supplementary Table 7). The results indicate that the procedure is safe and effectively reduces SMR up to 3 years.338 However, in the MITRA-FR trial,323336 MitraClip implantation had no impact on the primary endpoint of all-cause mortality or heart failure hospitalization at 12 months and 2 years compared to GDMT alone. In the COAPT trial,337 MitraClip implantation substantially reduced the primary endpoint of cumulative hospitalizations for heart failure, as well as several pre-specified secondary endpoints, including all-cause mortality at 2 years.
Subanalyses of the COAPT trial confirm the positive response to TEER in several patient subgroups;339340341342343 conversely, the effect of the interventional treatment was neutral throughout all subgroups in an echocardiographic subanalyses of the MITRA-FR trial.344
The conflicting results of these two trials have generated considerable discussion. These diverging results might be partially explained by effect size of the trials, differences in trial design, patient selection, echocardiographic assessment of SMR severity, use of medical therapy, and technical factors. Patients in COAPT demonstrated greater severity of SMR (EROA 41±15 mm2 vs. 31±10 mm2) and less LV dilatation (mean indexed LV end-diastolic volume 101±34 mL/m2 vs. 135±35 mL/m2) than those enrolled in MITRA-FR. Perhaps reflecting greater severity of SMR in relation to LV dimensions (‘disproportionate’ mitral regurgitation), patients in COAPT were overall more likely to benefit from TEER in terms of reduced mortality and heart failure hospitalization.345
Additional studies are needed to identify patients who will benefit the most from TEER.
Therefore, TEER should be considered in selected patients with severe SMR fulfilling the COAPT inclusion criteria,346347348 who receive optimal medical therapy supervised by a heart failure specialist and are as close as possible to the patients actually enrolled in the study. Optimization of the procedural result should also be pursued. In addition, TEER may be considered only in selected cases when the COAPT criteria are not fulfilled with the aim of improving symptoms and quality of life.349350351352353 In patients with less severe SMR (EROA <30 mm2) and advanced LV dilatation/dysfunction, the prognostic benefit of MitraClip remains unproven.323354355 Patients with end-stage LV and/or RV failure and no option for revascularization may be better served by cardiac transplantation or LV assist device implantation. Valve intervention is generally not an option when LVEF is <15%.247
The management of moderate ischaemic SMR in patients undergoing CABG remains an object of debate.322330 Surgery is more likely to be considered if myocardial viability is present and if comorbidity is low. Exercise-induced dyspnoea and a large increase in mitral regurgitation severity and SPAP favour combined surgery.
Transcatheter mitral valve repair systems other than TEER, as well as transcatheter mitral valve replacement devices, are currently the subject of intense investigation but clinical data are still limited.
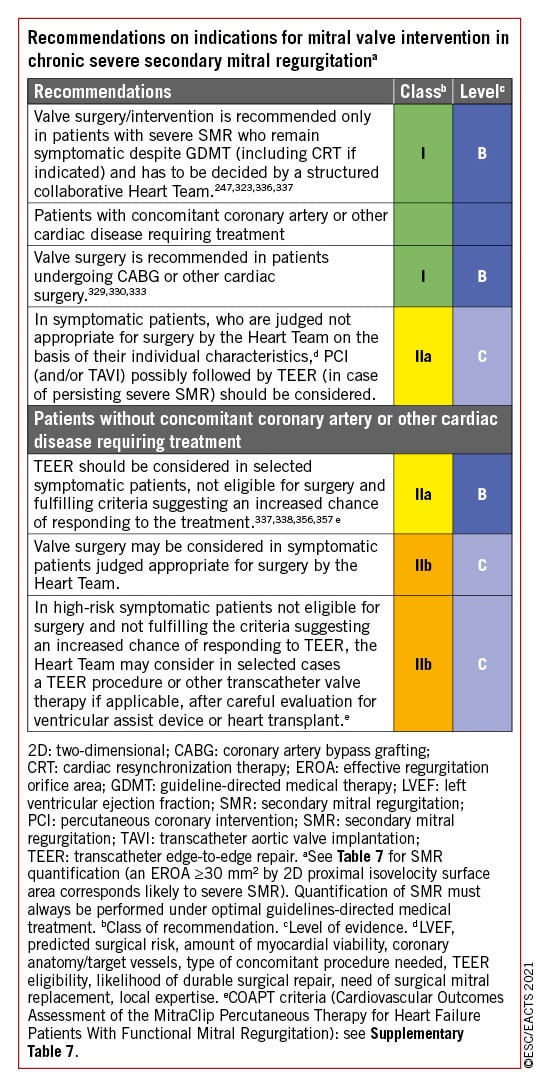
7 Mitral stenosis
Aetiology of mitral stenosis is mostly rheumatic or degenerative. Rheumatic fever is the most common cause of mitral stenosis worldwide. Its prevalence has greatly decreased in industrialized countries, but it remains a significant healthcare problem in developing countries and affects young patients.2267358 Degenerative mitral stenosis related to MAC is a distinct pathology and its prevalence significantly increases with age.359360 Both types of mitral stenosis are more frequent in females.361 In rare cases, mitral stenosis due to valve rigidity but without commissural fusion, may be related to chest radiation, carcinoid heart disease, or inherited metabolic diseases.
7.1 RHEUMATIC MITRAL STENOSIS
7.1.1 EVALUATION
Clinically significant mitral stenosis is defined by a mitral valve area (MVA) ≤1.5 cm2. Commissural fusion with thickening of the posterior leaflet is the most important mechanism of stenosis. Echocardiography is the preferred method for diagnosis, assessment of severity, and haemodynamic consequences of mitral stenosis. Valve area using 2D planimetry is the reference measurement of mitral stenosis severity, whereas mean transvalvular gradient and pulmonary pressures reflect its consequences and have a prognostic role.362 3D-TTE planimetry may have an additional diagnostic value. TTE usually provides sufficient information for routine management. Scoring systems have been developed to help assess suitability for percutaneous mitral commissurotomy (PMC; Supplementary Table 8),363364365 TOE should be performed to exclude LA thrombus before PMC or after an embolic episode, and to obtain detailed information on mitral anatomy (commissural zones and subvalvular apparatus) before intervention when TTE is suboptimal. Stress testing is indicated in patients with no symptoms or symptoms equivocal or discordant with the severity of mitral stenosis. Exercise echocardiography may provide objective information by assessing changes in mitral gradient and pulmonary artery pressure and is superior to DSE. Echocardiography plays an important role in the periprocedural monitoring of PMC and follow-up.
7.1.2 INDICATIONS FOR INTERVENTION
The type of treatment (PMC or surgery), as well as its timing, should be decided based on clinical characteristics, anatomy of valve and subvalvular apparatus, and local expertise.366367368369 In general, indication for intervention should be limited to patients with clinically significant (moderate-to-severe) rheumatic mitral stenosis (valve area ≤1.5 cm2) in whom PMC has had a significant impact on its management. In Western countries where incidence of rheumatic fever and number of PMC is low, this treatment should be restricted to expert operators in specialized centres to improve safety and procedural success rate.366 Efforts should be made to increase availability of PMC in developing countries where access to treatment is limited due to economic reasons.267 PMC should be considered as an initial treatment for selected patients with mild to moderate calcification or impaired subvalvular apparatus, but who have otherwise favourable clinical characteristics.360
The management of clinically significant rheumatic mitral stenosis is summarized in Figure 7 and the indications and contraindications for PMC are provided in the table of recommendations below, and Table 8.
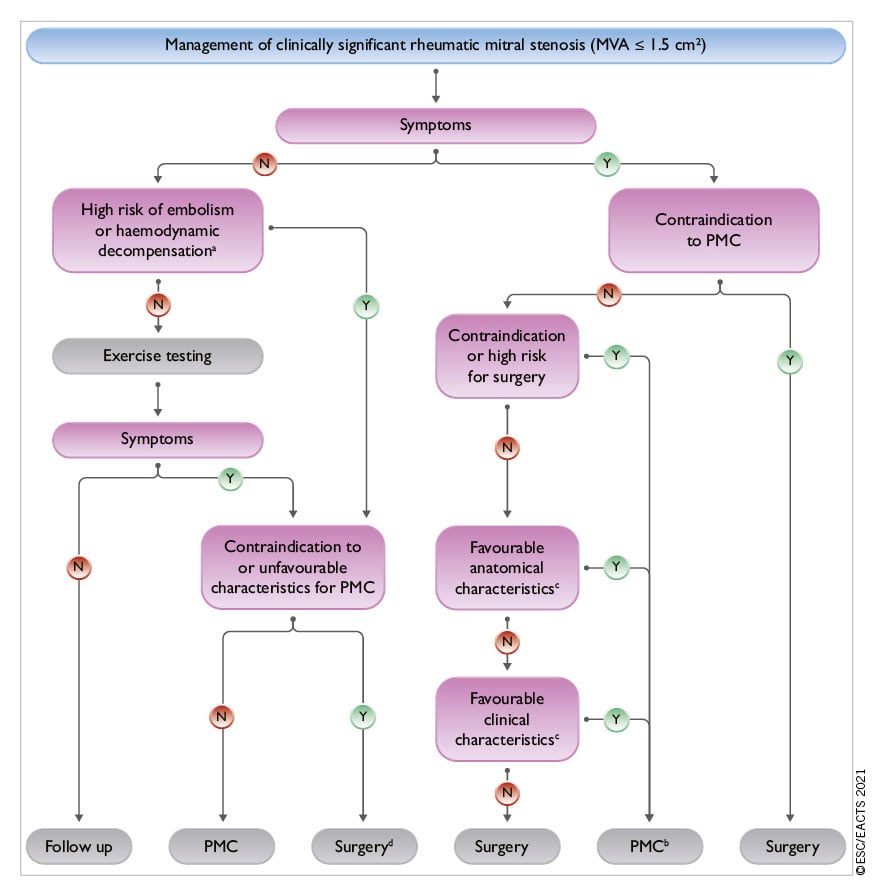
Figure 7. Management of clinically significant rheumatic mitral stenosis (MVA ≤1.5 cm2). AF: atrial fibrillation; LA: left atrium/left atrial; MVA: mitral valve area; NCS: non-cardiac surgery; PMC: percutaneous mitral commissurotomy. aHigh thromboembolic risk: history of systemic embolism, dense spontaneous contrast in the LA, new-onset AF. High-risk of haemodynamic decompensation: systolic pulmonary pressure >50 mmHg at rest, need for major NCS, desire for pregnancy. bSurgical commissurotomy may be considered by experienced surgical teams in patients with contraindications to PMC. cSee recommendations on indications for PMC and mitral valve surgery in clinically significant mitral stenosis in section 7.2. dSurgery if symptoms occur for a low level of exercise and operative risk is low.
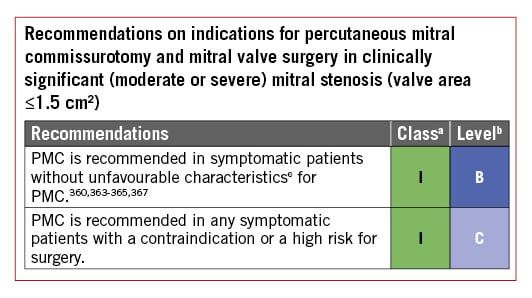
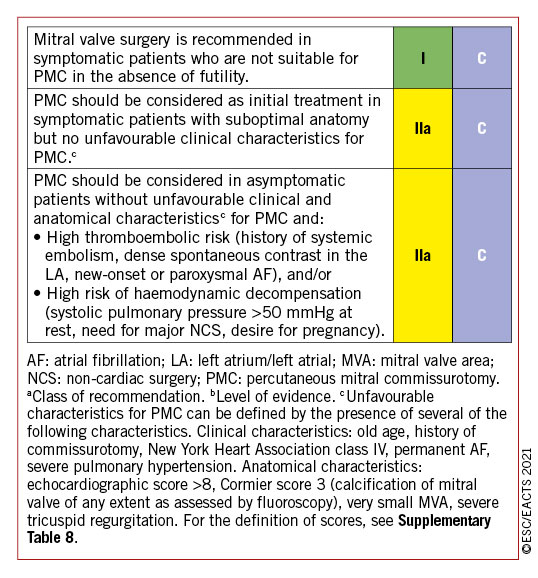
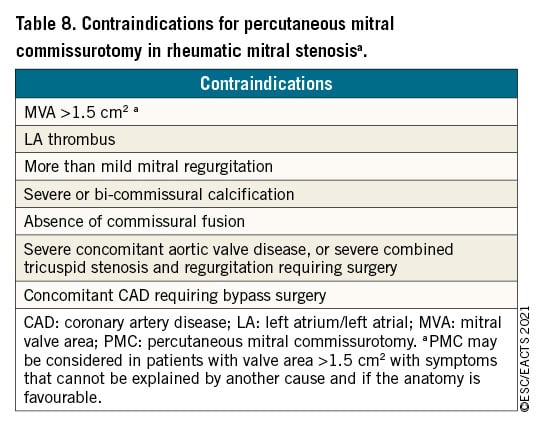
7.1.3 MEDICAL THERAPY
Diuretics, beta-blockers, digoxin, non-dihydropyridine calcium channel blockers and ivabradine can improve symptoms. Anticoagulation with vitamin K antagonist (VKA) with a target international normalized ratio (INR) between 2 and 3 is indicated in patients with AF. Patients with moderate-to-severe mitral stenosis and AF should be kept on VKA and not receive NOACs. Currently there is no solid evidence to support the use of NOACs in this setting370 and a randomized clinical trial is underway (INVICTUS VKA NCT 02832544). Neither cardioversion nor catheter pulmonary vein isolation are indicated before intervention in patients with significant mitral stenosis, as they do not durably restore sinus rhythm. If AF is of recent onset and the LA is only moderately enlarged, cardioversion should be performed soon after successful intervention, it should also be considered in patients with less than severe mitral stenosis. Amiodarone is most effective in maintaining the sinus rhythm after cardioversion. In patients in sinus rhythm, OAC is recommended when there has been a history of systemic embolism or a thrombus is present in the LA and should also be considered when TOE shows dense spontaneous echocardiographic contrast or an enlarged LA (M-mode diameter >50 mm or LA volume >60 mL/m2).
7.1.4 SERIAL TESTING
Asymptomatic patients with clinically significant mitral stenosis should be followed up yearly by clinical and echocardiographic examinations; and at longer intervals (2-3 years) in case of moderate stenosis. Follow-up of patients after successful PMC is similar to that of asymptomatic patients and should be more frequent if asymptomatic restenosis occurs.
7.1.5 SPECIAL PATIENT POPULATIONS
When symptomatic restenosis occurs after surgical commissurotomy or PMC, re-intervention in most cases requires valve replacement, but PMC can be proposed in selected candidates with favourable characteristics if the predominant mechanism is commissural refusion.369
In patients with severe rheumatic mitral stenosis combined with severe aortic valve disease, surgery is preferable when it is not contraindicated. The management of patients in whom surgery is contraindicated is difficult and requires a comprehensive and individualized evaluation by the Heart Team. In cases with severe mitral stenosis associated with moderate aortic valve disease, PMC can be performed to postpone the surgical treatment of both valves. In patients with severe tricuspid regurgitation, PMC may be considered in selected patients with sinus rhythm, moderate atrial enlargement, and severe functional tricuspid regurgitation secondary to pulmonary hypertension. In other cases, surgery on both valves is preferred.371
In the elderly population with rheumatic mitral stenosis when surgery is high risk, PMC is a useful option, even if as palliative care.364367368 Treatment of patients with low-gradient severe mitral stenosis (MVA ≤1.5 cm2, mean gradient <10 mmHg) is difficult, as these patients are older and have less optimal anatomy.372
7.2 DEGENERATIVE MITRAL STENOSIS WITH MITRAL ANNULAR CALCIFICATION
MAC is a distinct entity that differs from rheumatic mitral stenosis. Usually, these patients are elderly and may have significant comorbidities including disease of other valves. Overall, the prognosis is poor due to high-risk profile and technical anatomic challenges resulting from the presence of annular calcification.373 Between 9% and 15% of the general population may have MAC, with higher frequency in elderly patients (40%).67374375376 Furthermore, almost half of patients with aortic stenosis undergoing TAVI have MAC, and the disease is severe in 9.5% of cases.359377 Severe MAC may result in mitral stenosis (more frequently) or mitral regurgitation, or both.
7.2.1 EVALUATION
In patients with degenerative mitral stenosis and MAC, the echocardiographic evaluation of the disease severity is difficult and the usual parameters lack validation. Planimetry is less reliable due to diffuse calcium and irregular orifice. The mean transmitral gradient has been shown to have prognostic value.378 For the evaluation of severity, it is necessary to take into account the abnormalities of LA and LV compliance before indicating an intervention. If an intervention is planned, echocardiography is used for initial evaluation and CCT is necessary to assess the degree and location of calcification and to evaluate the feasibility of an intervention.379
7.2.2 INDICATIONS FOR INTERVENTION
Treatment options, including transcatheter and surgical approaches, are high-risk procedures and evidence from randomized trials is lacking. Even if the procedure is done successfully and the transvalvular gradient is reduced, due to low compliance of the LA and LV the mean atrial pressure may remain elevated.
In elderly patients with degenerative mitral stenosis and MAC, surgery is technically challenging and high risk.380 As there is no commissural fusion, degenerative mitral stenosis is not amenable to PMC.359 In symptomatic inoperable patients with suitable anatomy, preliminary experience showed that transcatheter mitral valve implantation (in mitral position, using an inverted balloon-expandable TAVI prosthesis), is feasible in selected patients with severe mitral stenosis, when performed by experienced operators after careful pre-planning using multimodality imaging.379 The largest case series reported to date included only 116 patients.381 However, operative mortality is high, in particular due to the risk of LVOT obstruction and mid-term results are less favourable compared to mitral valve-in-valve procedures.382383 The most recent case series show that results are improving owing to better patient selection and the use of different accesses, as well as concomitant or preventive measures such as alcohol septal ablation384 or laceration/resection of the anterior leaflet.385386387
Recently, a preliminary case series suggested that transcatheter mitral valve replacement using a dedicated prosthesis is feasible and can result in symptom improvement.388
8 Tricuspid regurgitation
Moderate or severe tricuspid regurgitation is observed in 0.55% of the general population and its prevalence increases with age, affecting about 4% of the patients aged 75 years or more.389 Aetiology is secondary in ≥90% of cases, due to pressure and/or volume overload mediated RV dilatation or enlarged right atrium and tricuspid annulus due to chronic AF. Secondary tricuspid regurgitation is associated with left-sided valvular or myocardial dysfunction in most cases, whereas it is isolated in 8.1% of subjects and independently related to mortality.389 Secondary tricuspid regurgitation may also develop late after left-sided valve surgery.390391
Causes of primary tricuspid regurgitation include infective endocarditis [especially in intravenous (i.v.) drug addicts], rheumatic heart disease, carcinoid syndrome, myxomatous disease, endomyocardial fibrosis, congenital valve dysplasia (e.g. Ebstein’s anomaly), thoracic trauma, and iatrogenic valve damage.
Atrial fibrillation induces annular remodelling even in the absence of left-heart disease.392 Cardiac implantable electronic device-lead implantation leads to progressive tricuspid regurgitation in 20-30% of the patients393394395 and predicts its progression over time.396
In patients with heart failure and reduced LVEF, secondary tricuspid regurgitation is a very frequent finding and is an independent predictor of clinical outcomes.397
8.1 EVALUATION
Tricuspid regurgitation should be evaluated first by echocardiography. In primary tricuspid regurgitation, specific abnormalities of the valve can be identified. In secondary tricuspid regurgitation, annular dilatation, along with RV and right atrium dimensions, as well as RV function should be measured, owing to their prognostic relevance.398 In experienced laboratories, RV strain27 and/or 3D measurements of RV volumes399400 may be considered to overcome the existing limitations of conventional RV function indices.102 When available, CMR is the preferred method to assess the RV400 due to its high accuracy and reproducibility.401
Echocardiographic evaluation of tricuspid regurgitation severity is based on an integrative approach considering multiple qualitative and quantitative parameters (Table 9). Due to the non-circular and non-planar shape of the regurgitant orifice, biplane vena contracta width should be considered in addition to the conventional 2D measurement.402 Similarly, underestimation of tricuspid regurgitation severity by the PISA method may occur.403 In case of inconsistent findings, the 3D vena contracta area may be evaluated, although diverging cut-offs have been reported.402404405406 Recently, a new grading scheme including two additional grades (‘massive’ and ‘torrential’) has been proposed407 and used in clinical studies on transcatheter interventions.408409 Studies showed an incremental prognostic value of the two additional grades (massive and torrential) in terms of mortality and rehospitalization for heart failure in patients with advanced disease.410411412

Alternatively, calculation of the tricuspid regurgitant volume by CMR using RV volumetry may be helpful.
Importantly, estimation of pulmonary pressures using Doppler gradient may be impossible or might underestimate the severity of pulmonary hypertension in the presence of severe tricuspid regurgitation, justifying cardiac catheterization to evaluate pulmonary vascular resistances.413
8.2 INDICATIONS FOR INTERVENTION
Severe tricuspid regurgitation is associated with impaired survival389414415416 and worsening heart failure.397417 In clinical practice, tricuspid valve interventions are underused and often initiated too late.418419420 Appropriate timing of intervention is crucial to avoid irreversible RV damage and organ failure with subsequent increased surgical risk421422 (see table of recommendations on indications for intervention in tricuspid valve disease in section 9 and Figure 8).
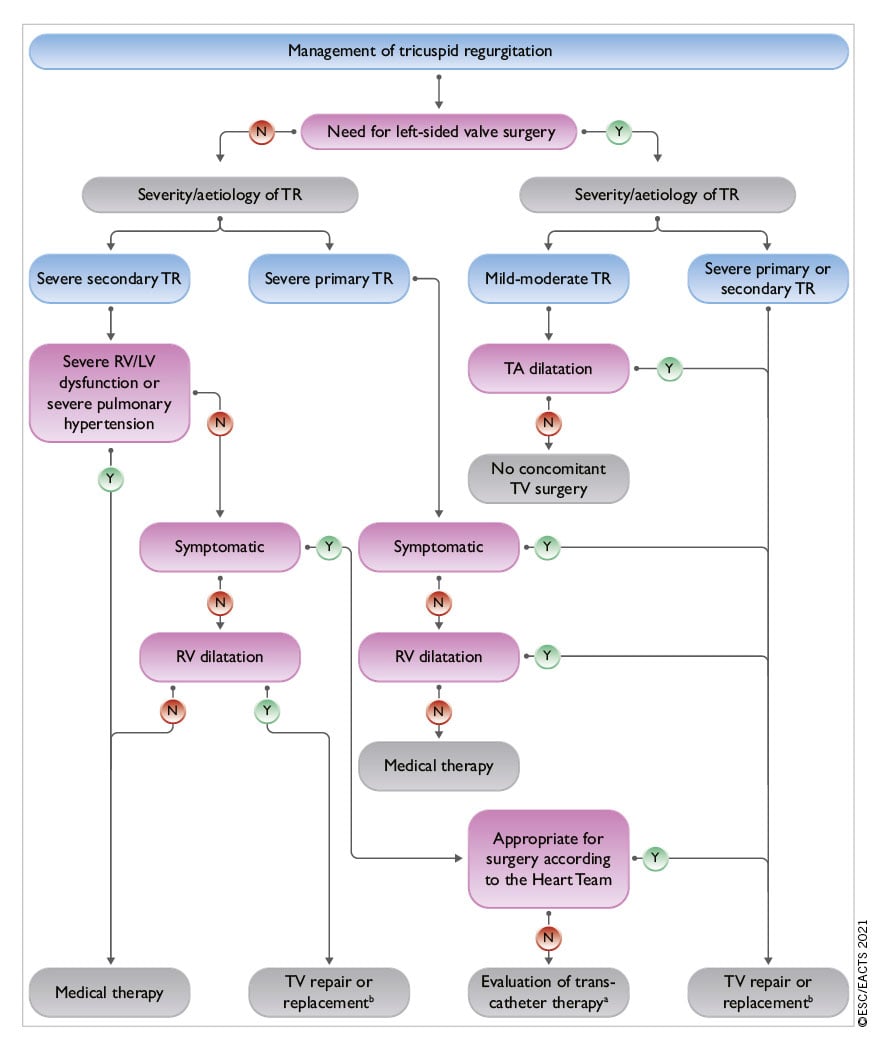
Figure 8. Management of tricuspid regurgitation. LV: left ventricle/left ventricular; RV: right ventricle/right ventricular; TA: tricuspid annulus; TR: tricuspid regurgitation; TV: tricuspid valve. aThe Heart Team with expertise in the treatment of tricuspid valve disease evaluates anatomical eligibility for transcatheter therapy including jet location, coaptation gap, leaflet tethering, potential interference with pacing lead. bReplacement when repair is not feasible.
Surgery is recommended in symptomatic patients with severe primary tricuspid regurgitation. In selected asymptomatic or mildly symptomatic patients who are appropriate for surgery, an intervention should also be considered when RV dilatation or declining RV function is observed. However, exact thresholds have not yet been defined.
According to observational data, tricuspid valve repair should be performed liberally during left-sided surgery in patients with secondary tricuspid regurgitation. Indeed, it does not increase operative risk, but promotes reverse remodelling of the RV and improves functional status when annular dilatation is present, even in the absence of severe tricuspid regurgitation.423424425426427
The benefit of surgical correction of isolated secondary tricuspid regurgitation compared to medical treatment is not well established428 and the procedure has a non-negligible risk of periprocedural mortality and morbidity when patients present late.429430431432 However, in carefully selected candidates, surgery can be performed safely with good long-term survival.418433 It should therefore be considered early in selected symptomatic patients appropriate for surgery, as well as in those with no or mild symptoms, RV dilatation and severe tricuspid regurgitation. Although a tricuspid annular pulmonary systolic excursion (TAPSE) <17 mm has been associated with worse prognosis in patients with secondary tricuspid regurgitation,398434 thresholds for severe RV dysfunction making intervention futile have not yet been defined.
Reoperation on the tricuspid valve in new-onset or worsening secondary tricuspid regurgitation after left-sided surgery carries a high procedural risk, possibly due to late referral and subsequent poor clinical condition.435 To improve prognosis, treatment of severe tricuspid regurgitation in this challenging scenario should be considered even in asymptomatic patients if there are signs of RV dilatation or decline in RV function (after exclusion of left-sided valve dysfunction, severe RV or LV dysfunction and severe pulmonary vascular disease/hypertension).
Whenever possible, annuloplasty with prosthetic rings is preferable to valve replacement,423430436 which should only be considered when the tricuspid valve leaflets are tethered and the annulus severely dilated. In presence of a cardiac implantable electronic device lead, the technique used should be adapted to the patient’s condition and the surgeon’s experience.437
TTVI are under clinical development. Early registry and study data demonstrated the feasibility to reduce tricuspid regurgitation using various systems, enabling either leaflet approximation [,408,438-440] direct annuloplasty,409441 or valve replacement,442443444 with subsequent symptomatic and haemodynamic improvement.445446 In a propensity-score-matched study comparing medical treatment to TTVI, all-cause mortality and rehospitalizations at 1 year were lower among the patients who received the interventional treatment.447 Several RCTs will investigate the efficacy of TTVI against medical treatment.
Therefore, TTVI may be considered by the Heart Team at experienced Heart Valve Centres in symptomatic, inoperable, anatomically eligible patients in whom symptomatic or prognostic improvement can be expected. For detailed anatomical evaluation, TOE and CCT may be preferred owing to higher spatial resolution.448449
8.3 MEDICAL THERAPY
Diuretics are useful in the presence of right heart failure. To counterbalance the activation of the renin-angiotensin-aldosterone system associated with hepatic congestion, the addition of an aldosterone antagonist may be considered.247 Dedicated treatment of pulmonary hypertension is indicated in specific cases. Although data are limited, rhythm control may help to decrease tricuspid regurgitation and contain annular dilatation in patients with chronic AF.450 Importantly, in the absence of advanced RV dysfunction or severe pulmonary hypertension, none of the above-mentioned therapies should delay referral for surgery or transcatheter therapy.
9 Tricuspid stenosis
Tricuspid stenosis is often combined with tricuspid regurgitation and most frequently of rheumatic origin. It is therefore usually associated with left-sided valve lesions, particularly mitral stenosis. Other causes are rare, including congenital, carcinoid and drug-induced valve diseases, Whipple’s disease, endocarditis, and large right atrial tumour.
9.1 EVALUATION
Echocardiography provides the most useful information. Tricuspid stenosis is often overlooked and requires careful evaluation. Echocardiographic evaluation of valve anatomy and subvalvular apparatus is important to assess valve reparability. No generally accepted grading of tricuspid stenosis severity exists, but a mean echocardiographic transvalvular gradient ≥5 mmHg at normal heart rate is considered indicative of significant tricuspid stenosis.362
9.2 INDICATIONS FOR INTERVENTION
Intervention on the tricuspid valve is usually performed concomitantly during procedures for left-sided valve disease in patients who are symptomatic despite medical therapy. Although the lack of pliable leaflet tissue is a main limitation for valve repair, the choice between repair and replacement depends on anatomy and surgical expertise. Owing to satisfactory long-term durability, biological prostheses are usually preferred over mechanical valves, which have a high risk of thrombosis.451
Percutaneous tricuspid balloon valvuloplasty has been performed in a limited number of cases, either alone or in combination with PMC. It frequently induces significant regurgitation and long-term results are lacking.452 It can be considered in rare cases with anatomically suitable valves, when tricuspid stenosis is isolated or additional mitral stenosis can also be treated interventionally (see recommendations on indications for PMC and mitral valve surgery in clinically significant mitral stenosis in section 7).
9.3 MEDICAL THERAPY
Diuretics are useful in the presence of heart failure symptoms but are of limited long-term efficacy.
10 Combined and multiple-valve diseases
Significant stenosis and regurgitation can be found on the same valve. Disease of multiple valves may be encountered in several conditions, particularly in rheumatic and congenital heart disease, but also less frequently in degenerative valve disease. There is a lack of data on combined or multiple-valve disease.453454455456457458459460 This does not allow for evidence-based recommendations. The general principles for the management of combined or multiple-valve disease are as follows:
- When either stenosis or regurgitation is predominant, management follows the recommendations concerning the predominant VHD. When the severity of both stenosis and regurgitation is balanced, indications for interventions should be based on symptoms and objective consequences rather than on the indices of severity of stenosis or regurgitation.453454455456 In this setting, Doppler pressure gradient reflects the global haemodynamic burden (stenosis and regurgitation) of the valve lesion.453
- Besides the separate assessment of each valve lesion, it is necessary to consider the interaction between the different valve lesions. As an illustration, associated mitral regurgitation may lead to underestimation of the severity of aortic stenosis, as decreased stroke volume due to mitral regurgitation lowers the flow across the aortic valve and hence the aortic gradient.453 This underlines the need to combine different measurements, including assessment of valve areas, if possible using methods that are less dependent on loading conditions, such as planimetry.457
- Indications for intervention are based on global assessment of the consequences of the different valve lesions (i.e. symptoms or presence of LV dilatation or dysfunction). Intervention can be considered for non-severe multiple lesions associated with symptoms or leading to LV impairment.453
- The decision to intervene on multiple valves should take into account the age, comorbidities, and risk of combined procedures, and should be made by the Heart Team after precise and comprehensive evaluation of valve lesions and their interactions with each other.453461 The risk of combined intervention should be weighed against the evolution of untreated valve disease and the inherent risk of subsequent intervention.
- The choice of surgical technique/interventional procedure should take into account the presence of the other VHD.453458459461
- When interventional procedures are considered, staged procedures may be preferable in cases with aortic stenosis and mitral regurgitation (see section 5.5). Improved 1-year survival after combined transcatheter treatment of mitral and tricuspid regurgitation has been reported compared to mitral regurgitation alone.263 PMC may delay surgery, in situations such as severe mitral stenosis associated with moderate aortic regurgitation.
The management of specific associations of VHD is detailed in the individual sections of this document.
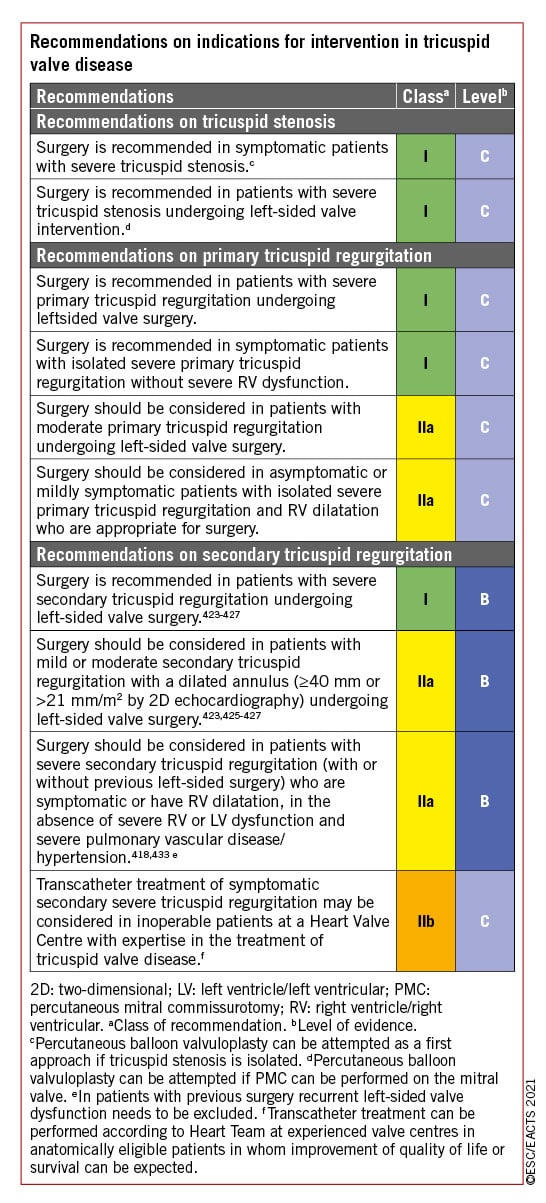
11 Prosthetic valves
11.1 CHOICE OF PROSTHETIC VALVE
Factors for valve selection are the patient’s life expectancy, lifestyle, and environmental factors, bleeding and thromboembolic risks related to anticoagulation, potential for surgical or transcatheter re-intervention, and, importantly, informed patient preference. Generally, biological heart valves (BHVs) should be preferred in patients with shorter anticipated survival or comorbidities that may lead to further surgical procedures, and those who are at increased risk for bleeding complications. Thromboembolic complications are less frequent in pregnant women with BHVs.
In a nationwide observational study, patients aged 45 to 54 with surgical aortic BHV implantation and those aged 40 to 70 years with surgical mitral BHV implantation had a significantly higher 15-year mortality than those with a mechanical heart valve (MHV). An analysis of patients 55 to 64 years of age showed no difference in mortality between aortic BHV and MHV prosthesis.462 However, an earlier systematic review463 and a recent meta-analysis464 of studies comparing aortic MHVs and BHVs showed a significant mortality reduction with MHVs in patients ≤60 and in those 50-70 years of age, respectively. All these studies are limited by their predominantly observational nature and missing information on the type of prostheses implanted. There is no new high-quality evidence supporting a decrease in the established age cut-off for prosthesis selection.
The best aortic valve substitute for younger adults remains unclear. In appropriately selected patients, replacement of the aortic valve using an autograft may be performed, with long-term survival rates and valve-related reoperation that are comparable to those achieved with a MHV, but high expertise in aortic root surgery is required.465 Strategies for patients with small aortic annulus include root enlargement and use of stentless valves. Although the use of sutureless and rapid-deployment aortic valves may reduce invasiveness, cross-clamp and cardiopulmonary bypass times, and potentially lower perioperative complications of SAVR, there is a lack of a large-scale randomized comparison on both short- and long-term safety, efficacy, and haemodynamic performance of this approach against conventional aortic valve replacement, which remains the gold standard of procedure.
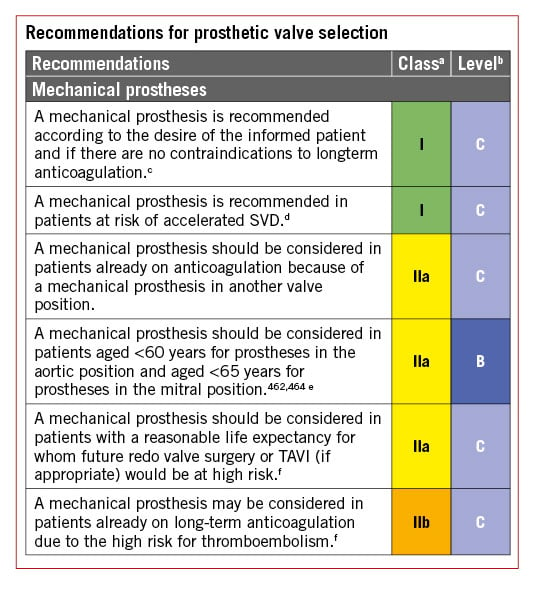

11.2 BASELINE ASSESSMENT AND FOLLOW-UP
All patients with prosthetic valves require lifelong follow-up to detect early deterioration in prosthetic function or ventricular function, or progressive disease of another heart valve.314 Clinical assessment should be performed yearly or as soon as possible if new cardiac symptoms occur. TTE should be performed if any new symptoms occur or if complications are suspected. After transcatheter, as well as surgical implantation of a BHV, echocardiography, including measurement of transprosthetic gradients, should be performed within 30 days after valve implantation (i.e. baseline), at 1 year, and annually thereafter.470 TOE should be considered if TTE is of poor quality and in all cases of suspected prosthetic dysfunction (especially if the prosthesis is in the mitral position) or endocarditis.314471 Cinefluoroscopy for MHVs and CCT scanning provide useful additional information if valve thrombus or pannus are suspected to impair valve function.314
11.3 ANTITHROMBOTIC MANAGEMENT
11.3.1 MECHANICAL PROSTHESES
11.3.1.1 Postoperative anticoagulation management
MHVs require lifelong treatment with VKA guided by the INR [.472,473] NOACs currently have no role in patients with MHVs.474 Treatment with VKA should be started on the first postoperative day in combination with bridging therapy [with therapeutic doses of either unfractionated heparin (UFH) or off-label use of low-molecular-weight heparin (LMWH)] until therapeutic INR is achieved.475 Similar safety and efficacy outcomes have been reported following bridging with either UFH or LMWH.476 Once a stable therapeutic INR is reached for ≥24 h, bridging can be discontinued. The postoperative risk of thromboembolism peaks about 1 month after implantation, but risks are substantially increased up to 6 months.477478 Long-term prevention of valve thrombosis and thromboembolism after MHV implantation involves effective antithrombotic medication and risk factor modification for thromboembolism.479
11.3.1.2 Target international normalized ratio
Target INR should be based upon prosthesis thrombogenicity and patient-related risk factors (Table 10).479 It is recommended to target a median INR value rather than a range to avoid considering extreme values in the target range as a valid target INR. High INR variability is a strong independent predictor of adverse events after valve replacement. Although some studies have supported lowering a target INR for aortic MHVs,480481 further evaluation in larger cohorts is warranted before updating current recommendations. The use of self-monitoring INR is associated with a lower rate of VKA-related complications in all ages.482 In a trial of lower intensity warfarin plus aspirin (INR 1.5-2.0) or standard warfarin plus aspirin (INR 2.0-3.0) after implantation of the On-X MHV in the aortic position, the similar safety of the two approaches was partly attributed to use of home INR monitoring and high degree of adherence among patients.481 Patient’s education plays an important role for achieving stable anticoagulation in the therapeutic range. Effective management of patients with unstable INR requires frequent in-clinic testing and dose titration. Because of the lack of good-quality evidence, pharmacogenetic testing cannot be recommended to guide the dosing of VKAs.
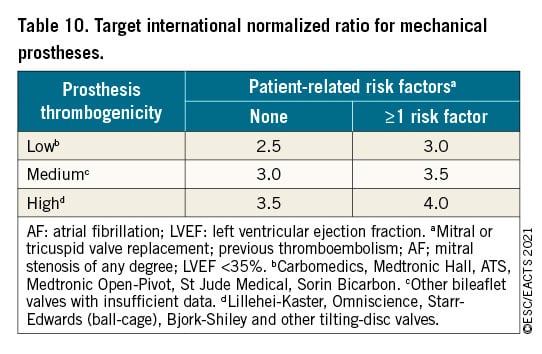
11.3.1.3 Management of vitamin K antagonist (VKA) overdose and bleeding
Bleeding increases exponentially with INR >4.5.483 In case of major and/or life-threatening bleeding and in patients who need to undergo urgent surgery, the VKA should be discontinued and 10 mg vitamin K should be administrated by slow i.v. infusion and repeated every 12 h if needed. Until the anticoagulation effect is reversed, administration of prothrombin complex concentration (PCC) and/or fresh frozen plasma (FFP) therapy should be initiated according to body weight and pre-treatment INR. The efficacy should be monitored by re-check of INR at 30 min and every 4-6 h until normalization. The optimal time to restart anticoagulation should be discussed in relation to location of the bleeding event and interventions performed to stop bleeding and/or to treat an underlying cause.484
In the absence of bleeding, the use of PCC and/or FFP therapy is not recommended and the decision to start vitamin K should be individualized. In asymptomatic patients with INR >10, the VKA must be stopped and oral vitamin K (2.5-5 mg) prescribed, while the INR must be monitored on a daily base for 2 weeks. Multiple RCTs in patients with INR between 4.5 and 10 suggest no difference in bleeding events with vitamin K vs. placebo.483485 Therefore, in such patients, warfarin should be stopped temporarily, and a small dose of oral vitamin K (1-2 mg) can be considered on an individual basis balancing between the risks. Finally, asymptomatic patients with INR <4.5 require careful down-titration and/or skipping one or more doses. In all patients with MHVs, VKAs must be resumed once the INR achieves the therapeutic range or is slightly elevated.
11.3.1.4 Combination of oral anticoagulation (OAC) with antiplatelet drugs
The addition of low-dose (75-100 mg) acetylsalicylic acid (ASA) to VKA may reduce the incidence of thromboembolism at the cost of bleeding.477 Therefore, addition of antiplatelets to VKAs should be reserved for patients at very high risk of thromboembolism where advantages clearly outweigh the risks.486487 In patients with thromboembolism despite adequate INR, low dose (75-100 mg) ASA should be added to VKAs. Management of oral antithrombotic therapy in patients with CAD is summarized in Supplementary Figure 2.
11.3.1.5 Interruption of anticoagulant therapy for planned invasive procedures
In patients with MHVs, preoperative bridging with UFH or LMWH before surgery imposes a risk of perioperative bleeding while interrupting anticoagulation results in an increased risk of thromboembolism.488 Therefore, anticoagulation in patients with MHVs undergoing elective NCS requires careful management by multidisciplinary consensus.478489 For minor surgical procedures (e.g. dental, cataract, skin incision) in which blood loss is usually small and easily controlled, it is recommended that OAC is not interrupted. Major surgeries require temporary interruption and therapeutic bridging with either UFH or LMWH, aiming for an INR <1.5 (Supplementary Figure 3). Fondaparinux should not be routinely used for bridging, but may have a role in patients with history of heparin-induced thrombocytopenia.490
11.3.2 BIOPROSTHESES
11.3.2.1 Patients with no baseline indication to oral anticoagulation (OAC)
Surgical bioprostheses: The optimal antithrombotic strategy early after surgical implantation of an aortic BHV remains controversial due to lack of high-quality evidence. Multiple observational studies support the use of VKAs to reduce the risk of thromboembolism.491492493 A small randomized trial found that VKA for 3 months significantly increased major bleeding compared with ASA, without reducing the rate of deaths or thromboembolic events, but the statistical power was low for demonstrating a thrombotic benefit.494 VKA for 3 months should be considered in all patients with a mitral or tricuspid BHV and ASA or VKA should be considered for 3 months after surgical implantation of an aortic bioprosthesis.
Transcatheter bioprostheses: A meta-analysis of three small RCTs showed a significant increase in major or life-threatening bleeding with dual antiplatelet therapy (DAPT) over ASA at 30 days, with no difference in ischaemic outcomes.495 Consistently, the more recent POPular TAVI trial (cohort A) found reduced bleeding and the composite of bleeding or thromboembolic events with ASA compared with DAPT.496 A randomized trial was halted prematurely due to safety concerns with a rivaroxaban-based regimen as compared with DAPT, including a higher risk of death or thromboembolic complications and a higher risk of bleeding.497 There is a lack of data on the management of antithrombotic therapy after implantation of transcatheter mitral BHVs (e.g. valve-in-valve or valve-in-ring) for which 3 months of VKA is commonly prescribed.498
11.3.2.2 Patients with baseline indication to oral anticoagulation (OAC)
Surgical bioprostheses: OAC is recommended lifelong for patients with surgical BHVs who have other indications for anticoagulation. The evidence supporting the use of NOACs in preference to VKA has increased since the publication of the 2017 VHD Guidelines. In the RIVER trial, including patients with AF and a BHV in the mitral position, the NOAC rivaroxaban was non-inferior to warfarin with respect to a net benefit endpoint at 12 months.499 The benefit of NOAC was consistent among subgroups. However, only 20% of patients were enrolled in the trial before the third postoperative month, which raises a note of caution and calls for additional data in this particular subgroup. In the small ENAVLE trial (N = 220), including patients with and without AF, edoxaban was non-inferior to warfarin for preventing thromboembolism and the occurrence of major bleeding in the first 3 months after aortic or mitral surgical bioprosthetic valve implantation or repair, which warrants confirmation in larger investigations.500
Transcatheter bioprostheses: In the POPular TAVI trial (cohort B), the incidence of bleeding over a period of 1 month or 1 year was lower with OAC than with OAC plus clopidogrel.501 OAC alone was non-inferior to OAC plus clopidogrel with respect to ischaemic events, but the non-inferiority margin was large. An observational study suggested that there is a higher risk of ischaemic events at 1 year with NOACs compared with VKAs, after adjustment for potential confounders.502 Randomized trials comparing NOACs vs. VKAs are ongoing (NCT02943785, NCT02664649). Data on the management of antithrombotic therapy after transcatheter mitral or tricuspid valve implantation are scant.498


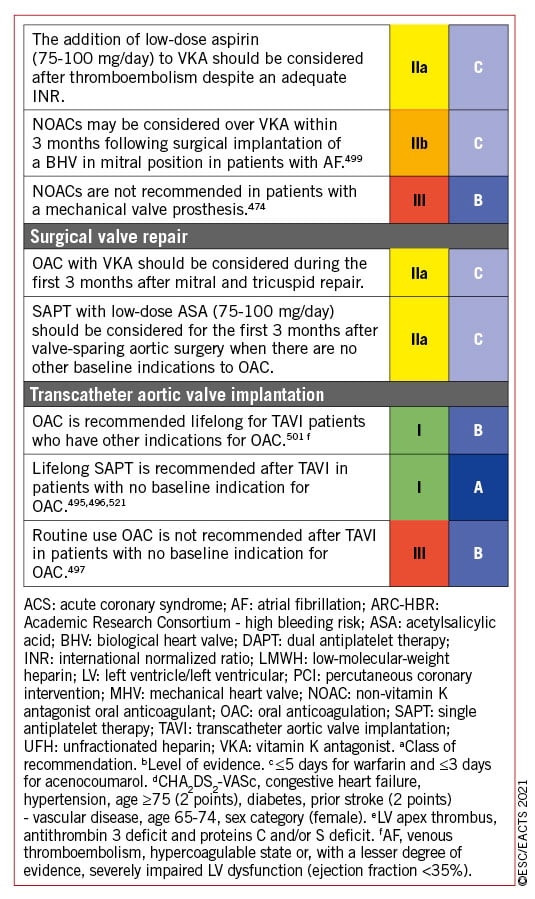
11.3.3 VALVE REPAIR
Observational data suggest comparable risk of thromboembolism with ASA or VKAs following mitral valve repair,503 but randomized data are lacking. The high incidence of new-onset AF and its recurrence, the thrombogenic tendency of the non-endothelialized repair components, and a relatively high rate of patients who are resistant to ASA establish VKAs as a preferable option for the initial period (e.g. 3 months). However, the potential for bleeding complications in the postoperative phase dictates careful patient selection.
The management of antithrombotic treatment after prosthetic valve implantation or valve repair is summarized in the table of recommendations for management of antithrombotic therapy after prosthetic valve implantation or valve repair and in Figure 9.
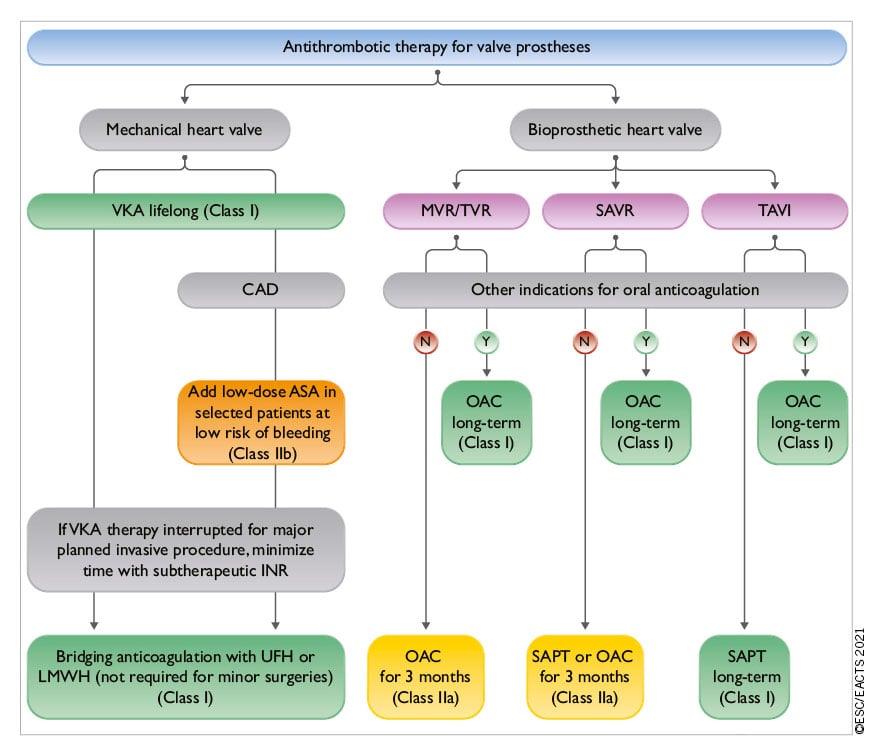
Figure 9. Antithrombotic therapy for valve prostheses. AF: atrial fibrillation; ASA: acetylsalicylic acid; CAD: coronary artery disease; DAPT: dual antiplatelet therapy; INR: international normalized ratio; LMWH: low-molecular-weight heparin; LV: left ventricle/left ventricular; MHV: mechanical heart valve; MVR: mitral valve replacement or repair; OAC: oral anticoagulation; SAPT: single antiplatelet therapy; SAVR: surgical aortic valve replacement; TAVI: transcatheter aortic valve implantation; TVR: tricuspid valve replacement or repair; UFH: unfractionated heparin; VKA: vitamin K antagonist. Colour coding corresponds to class of recommendation.
11.4 MANAGEMENT OF PROSTHETIC VALVE DYSFUNCTION AND COMPLICATIONS
11.4.1 STRUCTURAL VALVE DETERIORATION
Definitions of SVD and bioprosthetic valve failure (BVF) were standardized by recent consensus.470522 The comparative durability of TAVI and SAVR BHVs must be ascertained at longer term. Reversible causes of BVF (e.g. endocarditis, thrombosis) should be excluded, and considerations on timing of dysfunction (e.g. for BHV obstruction, mismatch in early phases, thrombosis in later phases) and location of malfunction (e.g. endocarditis or SVD in case of central regurgitation, endocarditis or anatomical/technical factors in case of paravalvular regurgitation) may reveal the most plausible underlying cause and guide clinical decision making.
Percutaneous balloon interventions should be avoided in the treatment of stenotic left-sided bioprostheses. Transcatheter valve-in-valve implantation is an option for treating degenerated BHVs in patients with increased surgical risk.227523524525 Redo-TAVI is a safe and feasible option in selected patients, but the risk of PPM in small valves and that of coronary occlusion, as well the possibility for future access to the coronary arteries need to be considered.229526527528 Experience is mostly in aortic BHVs and remains limited for BHVs in the mitral position and even more so in the tricuspid position529530531532 for which valve-in-valve procedures may be reasonable in patients at increased surgical risk.531533 Valve-in-ring mitral procedures are also acceptable in selected candidates, while the role of valve-in-ring tricuspid procedures remains uncertain. It is necessary for the Heart Team to discuss every patient and choose the best individualized approach. Careful pre-procedural planning is needed to minimize the risk of coronary artery obstruction and enable future coronary re-access in aortic BHV re-interventions if necessary. For mitral re-interventions the risk of LVOT obstruction should be carefully evaluated.534
11.4.2 NON-STRUCTURAL VALVE DYSFUNCTION
11.4.2.1 Patient-prosthesis mismatch
Patient-prosthesis mismatch (PPM) significantly decreases long-term survival, correlates with SVD and increases readmission rates for both heart failure and reoperation.535536537 Efforts to prevent PPM should receive more emphasis to improve long-term survival after either SAVR or TAVI.538
11.4.2.2 Paravalvular leak and haemolysis
Blood tests for haemolysis should be part of routine follow-up after valve replacement. Diagnosis of haemolytic anaemia requires TOE to detect paravalvular leaks for prostheses in the mitral position if TTE is not contributory. Reoperation is recommended if the paravalvular leak is related to endocarditis or causes haemolysis requiring repeated blood transfusions or leading to severe symptoms. Transcatheter closure of a paravalvular leak is feasible, but experience is limited and there is presently no conclusive evidence to show consistent efficacy.539 Transcatheter closure of paravalvular leaks should be considered for anatomically suitable paravalvular leaks in candidates selected by the Heart Team.540 Medical therapy (including iron supplementation, beta-blockers, and erythropoietin) is indicated in patients with severe haemolytic anaemia when contraindications to surgical or transcatheter closure are present.540
11.4.3 ENDOCARDITIS
The management of patients with endocarditis should follow the relevant guidelines.4
11.4.4 THROMBOSIS
11.4.4.1 General comments
Obstructive valve thrombosis should be suspected promptly in any patient with any type of prosthetic valve who presents with recent dyspnoea or an embolic event. The diagnosis should be confirmed by TTE and TOE, cinefluoroscopy, or CCT if promptly available.268314 Valve thrombosis occurs mainly in MHVs. However, cases of thrombosis of BHVs have also been reported after surgery or transcatheter valve implantation.541 Thrombus on BHVs can present as hypo-attenuated leaflet thickening (HALT) with relatively normal leaflet motion, HALT with reduced leaflet motion but normal gradients, and clinical valve thrombosis with elevated gradients. Distinguishing between thrombus and pannus by means of CCT is important to guide decision making.
11.4.4.2 Valve thrombosis
The management of MHVs thrombosis is high risk, whatever the option taken. Fibrinolysis carries risks of bleeding, systemic embolism, and recurrent thrombosis.542 Emergency valve replacement is recommended for obstructive prosthetic valve thrombosis in critically ill patients without a contraindication to surgery. Management of non-obstructive thrombosis of an MHV depends mainly on the occurrence of a thromboembolic event and the size of the thrombus. Surgery should be considered for a large (>10 mm) non-obstructive prosthetic valve thrombus that is complicated by embolism or persists despite optimal anticoagulation.543 Fibrinolysis may be considered if surgery is not an option or is very high risk for the treatment of thrombosis of right-sided prostheses, but carries a risk of bleeding and thromboembolism. Anticoagulation using a VKA and/or UFH is the first-line treatment of BHV thrombosis. Because BHV thrombosis is associated with recurrence and early prosthetic degeneration, indefinite anticoagulation should be considered after a confirmed episode, but this strategy must be balanced against an increased risk of bleeding544545 (Figure 10).
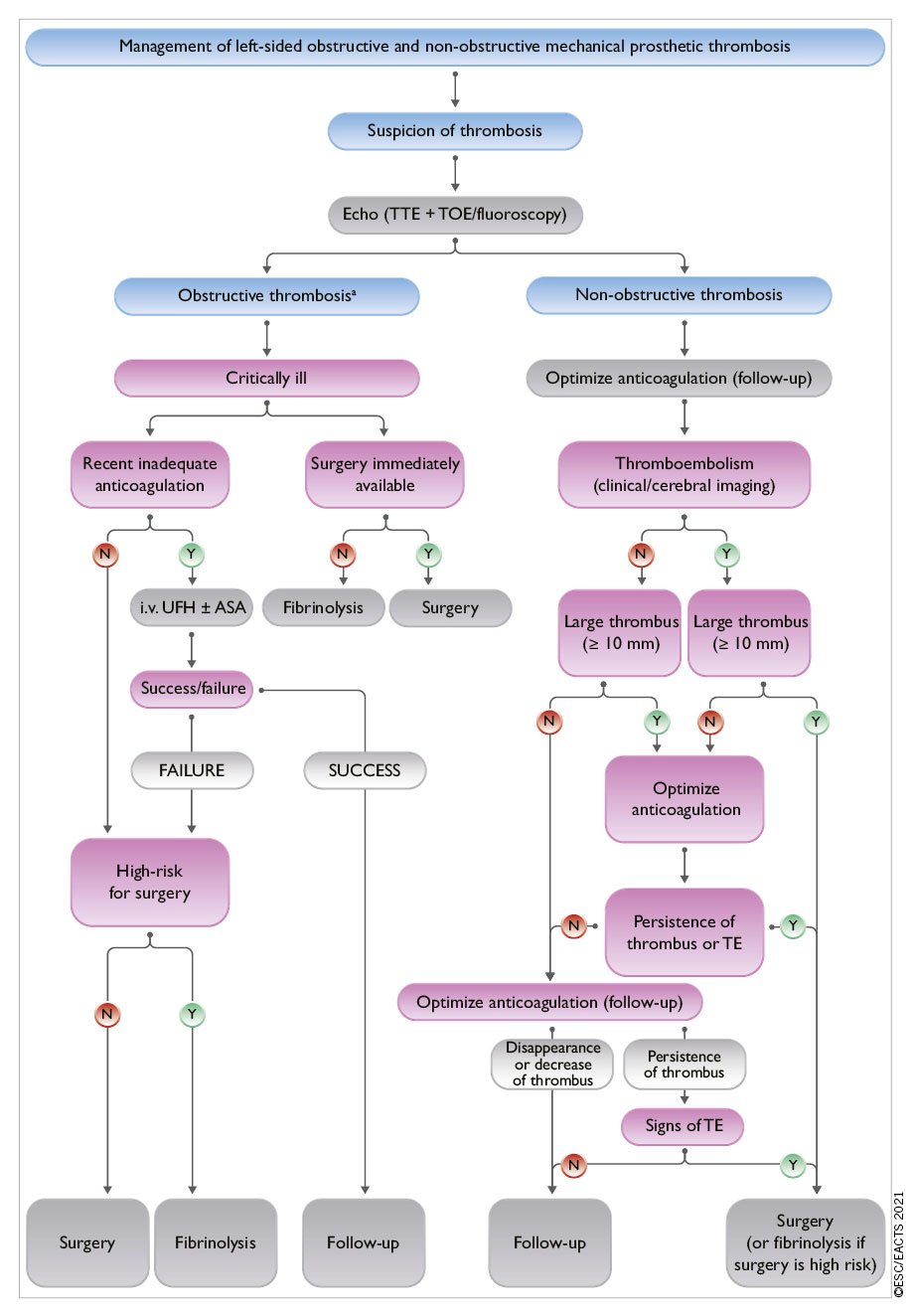
Figure 10. Management of left-sided obstructive and non-obstructive mechanical prosthetic thrombosis. ASA: acetylsalicylic acid; CCT: cardiac computed tomography; i.v.: intravenous; TOE: transoesophageal echocardiography; TE: thromboembolism; TTE: transthoracic echocardiography; UFH: unfractionated heparin. Risk and benefits of both treatments should be individualized. The presence of a first-generation prosthesis is an incentive to surgery. aRefer to recommendations for the imaging assessment of prosthetic heart valves. Evaluation generally includes TTE plus TOE or CCT and occasionally fluoroscopy.
11.4.4.3 Subclinical leaflet thrombosis
HALT is detected by CCT in 12.4% and 32.4% of TAVI patients on OAC or DAPT at 3 months, respectively.546 The clinical significance of these findings is uncertain. Selective use of oral anticoagulants in patients with confirmed HALT and restricted leaflet motion with elevated gradients should be considered.
11.4.5 HEART FAILURE
Heart failure after valve surgery should lead to a quick search for SVD or PPM, deterioration of repair, LV dysfunction, or progression of another valve disease. Non-valvular-related causes such as CAD, hypertension, or sustained arrhythmias should also be considered. The management of patients with heart failure should follow the relevant guidelines and consensus documents.142247

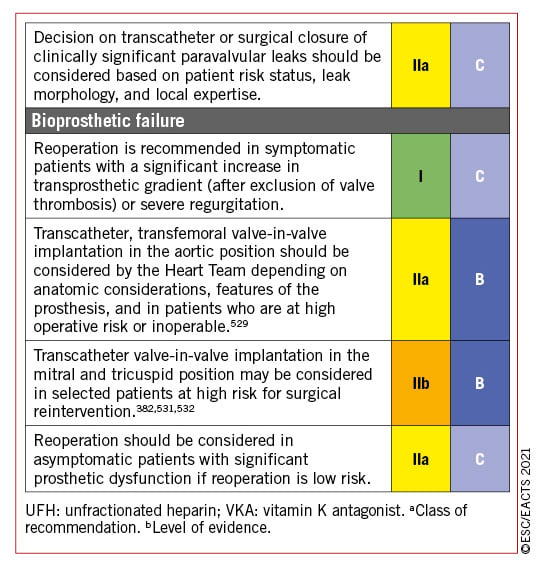
12 Management during non-cardiac surgery
Cardiovascular morbidity and mortality are increased in patients with VHD who undergo NCS. Symptomatic severe aortic stenosis or mitral stenosis may require valve replacement or percutaneous intervention before NCS. A detailed description of recommendations in the setting is available in specific ESC Guidelines.489
12.1 PREOPERATIVE EVALUATION
Patient and surgical specific factors dictate the strategy.489548549 The cardiologist provides recommendations on pre- and perioperative management, surveillance, and continuation of chronic cardiovascular medical treatment. Echocardiography should be performed in any patient with VHD requiring NCS. Determination of functional capacity is a pivotal step in preoperative risk assessment, measured either by ability to perform activities in daily life or by exercise test. The decision for management should be taken after multidisciplinary discussion involving cardiologists, surgeons, and cardiac anaesthesiologists, as well as the team who will be in charge of NCS.
Patients receiving anticoagulation treatment should be managed as discussed in section 11.
12.2 SPECIFIC VALVE LESIONS
12.2.1 AORTIC STENOSIS
In patients with severe aortic stenosis, urgent NCS should be performed under careful haemodynamic monitoring. In case of high risk of NCS, balloon valvuloplasty may be considered before NCS.549 Management related to elective NCS depends on the presence of symptoms and the type of surgery.489549550551552553 In symptomatic patients, aortic valve procedure should be considered before NCS. The type of procedure, TAVI or SAVR, is decided by the Heart Team. In asymptomatic patients, elective NCS, if at low to moderate risk, can be performed safely, albeit with a risk of worsening heart failure.489552553 If NCS implies large volume shifts, aortic valve procedure (TAVI or SAVR) should be considered first according to the Heart Team’s decision (Figure 11).
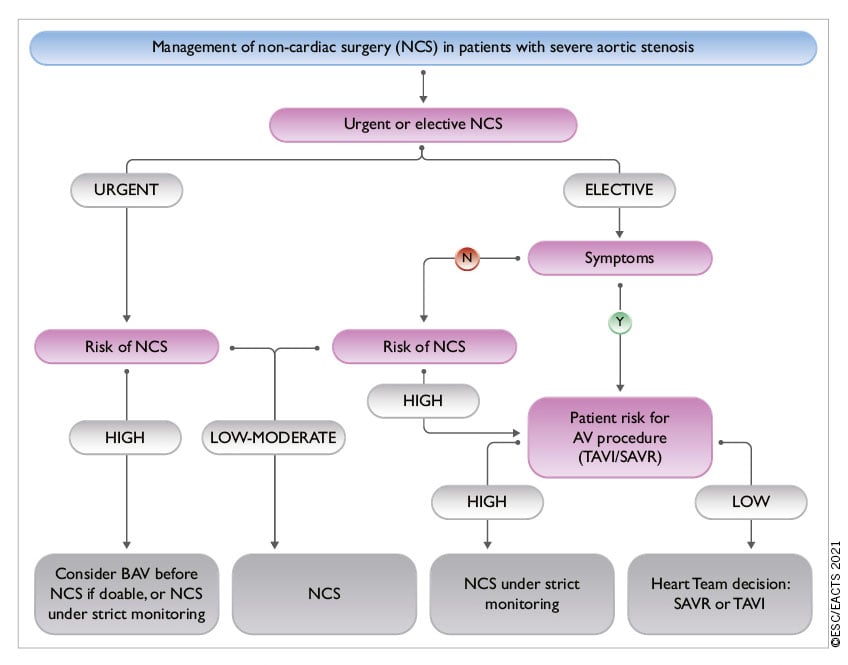
Figure 11. Management of non-cardiac surgery (NCS) in patients with severe aortic stenosis. AV: aortic valve; BAV: balloon aortic valvuloplasty; NCS: non cardiac surgery; SAVR: surgical aortic valve replacement; TAVI: transcatheter aortic valve implantation.
12.2.2 MITRAL STENOSIS
NCS can be performed safely in patients with non-significant mitral stenosis (valve area >1.5 cm2) and in asymptomatic patients with significant mitral stenosis and an SPAP <50 mmHg. In symptomatic patients or in patients with SPAP >50 mmHg, correction of mitral stenosis, by means of PMC whenever possible, should be attempted before NCS if it is high risk.
12.2.3 AORTIC AND MITRAL REGURGITATION
NCS can be performed safely in asymptomatic patients with severe mitral regurgitation or aortic regurgitation and preserved LV function. The presence of symptoms or LV dysfunction should lead to consideration of valvular surgery, but this is seldom needed before NCS. If LV dysfunction is severe (ejection fraction <30%) and/or SPAP is >50/60 mmHg, NCS should be performed only if strictly necessary and after optimization of medical therapy for heart failure.
12.3 PERIOPERATIVE MONITORING
Heart rate control (particularly in mitral stenosis) and careful fluid management (particularly in aortic stenosis) are needed. TOE monitoring may be considered.
13 Management during pregnancy
Detailed guidelines on the management of cardiovascular disease during pregnancy are available in another document.554 The decision for management before and during pregnancy should be taken after multidisciplinary discussion in the pregnancy Heart Team involving cardiologists, cardiac surgeons, obstetricians, neonatologists, and anaesthesiologists.
13.1 MANAGEMENT BEFORE PREGNANCY
Valve disease should be evaluated before pregnancy and treated if necessary.554555
Pregnancy should be discouraged, and intervention should be recommended before pregnancy in the following cases:
- Patients with mitral stenosis and a valve area <1.5 cm² (especially if <1.0 cm²).554556
- All symptomatic patients with severe AS or asymptomatic patients with impaired LV function (LVEF <50%) or an abnormal exercise test should be counselled against pregnancy, and surgery should be performed pre-pregnancy.554557
- Women with Marfan syndrome and an aortic diameter >45 mm should be strongly discouraged from becoming pregnant without prior aortic repair because of the high risk of aortic dissection. Although an aortic diameter <40 mm is rarely associated with aortic dissection, a completely safe diameter does not exist. With an aortic diameter between 40 and 45 mm, previous aortic growth and family history are important for advising pregnancy with or without aortic repair.558 Although the actual risk of dissection is not well documented in the setting of bicuspid valves, counselling against pregnancy is recommended in the setting of aortic diameters >50 mm (>27 mm² BSA).559 Finally, an aortic diameter >25 mm/m² BSA in Turner syndrome and all patients with vascular Ehlers-Danlos syndrome are also contraindications for pregnancy.
In women considering pregnancy and requiring heart valve replacement, it is recommended to choose the prosthesis in consultation with a pregnancy Heart Team.554560
Pregnancy in women with a mechanical valve, especially in the mitral position, is associated with a high risk of maternal and foetal complications,554561 which should be carefully discussed with the patient and family.
13.2 MANAGEMENT DURING PREGNANCY
13.2.1 PATIENTS WITH NATIVE VALVE DISEASE
Moderate or severe mitral stenosis with a valve area <1.5 cm² in pregnant women is usually poorly tolerated. PMC should be considered in severely symptomatic patients [New York Heart Association (NYHA) class III-IV] and/or those with SPAP >50 mmHg despite optimal therapy. PMC should preferably be performed after the 20th week of pregnancy in experienced centres.554
In patients who are severely symptomatic despite medical therapy, BAV for severe aortic stenosis can be undertaken by an experienced operator.557 TAVI is a promising alternative, but experience during pregnancy is very limited.554
Surgery under cardiopulmonary bypass is associated with a foetal mortality rate of 15-56%562 and should be restricted to the rare conditions that threaten the mother’s life if transcatheter intervention is not possible or has failed. Valve replacement should be considered after early delivery by caesarean section.
Caesarean section is recommended for patients with severe mitral or aortic stenosis, ascending aortic diameter >45 mm, severe pulmonary hypertension, or if delivery starts while treated with a VKA or <2 weeks after discontinuation of a VKA.
13.2.2 MECHANICAL PROSTHESIS
It is recommended to manage pregnancy in patients with MHV in a centre with a pregnancy Heart Team.554
Therapeutic anticoagulation during pregnancy is of utmost importance to avoid complications in these patients, keeping in mind that no anticoagulation regimen is ideal and management will require a careful balance between maternal and foetal risks.
In patients requiring <5 mg/day warfarin, oral anticoagulants throughout pregnancy and a change to UFH before delivery is favoured. In patients requiring higher doses, switching to LMWH during the first trimester with strict anti-Xa monitoring (therapeutic range 0.8-1.2 IU/mL, aortic valve prosthesis; and 1.0-1.2 IU/mL, mitral and right sided valve prosthesis) and the use of oral anticoagulants afterwards is favoured with a change to UFH before delivery.554
14 Key messages
GENERAL COMMENTS
1. Precise evaluation of the patient’s history and symptomatic status, as well as proper physical examination, are crucial for the diagnosis and management of VHD.
2. Echocardiography is the key technique to diagnose VHD and assess its severity and prognosis. Other non-invasive investigations such as CMR, CCT, fluoroscopy, and biomarkers provide important additional information in selected patients. Stress testing should be widely used in asymptomatic patients. Invasive investigation, beyond preoperative coronary angiography, is restricted to situations where non-invasive evaluation is inconclusive.
3. Decision making in elderly patients requires the integration of multiple parameters, including estimation of life expectancy and anticipated quality of life, evaluation of comorbidities, and general condition (including frailty).
4. Decision making in asymptomatic patients weighs the risk of intervention against the expected natural history of VHD. Stress testing should be liberally performed.
5. Informed patient’s expectations and values are an important part of the decision-making process.
6. Interventions (surgery or transcatheter) are indicated in symptomatic patients (spontaneous or exercise induced) in the absence of futility. In selected asymptomatic patients, presence of predictors of rapid symptom progression justifies early intervention when procedural risk is low.
7. Heart Valve Centres with multidisciplinary Heart Teams, Heart Valve Clinics, comprehensive equipment, and sufficient volumes of procedures are required to deliver high-quality care and provide adequate training.
8. Careful follow-up of symptomatic status, LV/RV size, and function is mandatory in asymptomatic patients with severe VHD if an intervention is not yet indicated.
9. In patients with AF, NOACs are contraindicated in patients with clinically significant mitral stenosis or mechanical valves. For stroke prevention in patients who are eligible for OAC, NOACs are recommended in preference to VKAs in patients with aortic stenosis, aortic and mitral regurgitation, or aortic bioprostheses >3 months after implantation.
AORTIC REGURGITATION
10. The evaluation of aortic regurgitation requires careful assessment of potentially associated aortic dilatation to guide the timing and type of surgery.
AORTIC STENOSIS
11. Diagnosis of severe aortic stenosis requires integrative evaluation of pressure gradients (the most robust measurements), AVA, extent of valve calcification, flow conditions, and LV function.
12. Selection of the most appropriate mode of intervention by the Heart Team should take into account clinical characteristics (age and estimated life expectancy, general condition), anatomical characteristics, the relative risks of SAVR and TAVI, the feasibility of transfemoral TAVI, local experience and outcome data, as well as informed patient preference.
MITRAL REGURGITATION
13. Regarding imaging, routine quantification of EROA is an important part of the integrative evaluation for quantification and risk stratification in patients with PMR. 3D transoesophageal echocardiography is more accurate than 2D echocardiography for defining the underlying mechanism of PMR. CMR is useful when echocardiographic evaluation of severe PMR grade is inconclusive.
14. Surgical mitral valve repair is the preferred method of treatment in PMR if a durable repair can be achieved. TEER is a safe but less efficacious alternative that may be considered in patients with contraindications for surgery or high operative risk.
15. In patients with severe SMR, GDMT (including CRT if indicated) should be the first step. If the patient remains symptomatic: mitral surgery is recommended concomitantly in patients with an indication for CABG or other cardiac surgery. Isolated valve surgery may be considered in selected patients. TEER should be considered in patients not eligible for surgery and fulfilling criteria indicating an increased chance of responding to the treatment. Circulatory support devices, cardiac transplantation, or palliative care should be considered as an alternative in patients with end-stage LV and/or RV failure.
MITRAL STENOSIS
16. PMC is currently the standard of care in patients with severe rheumatic mitral stenosis and favourable valve anatomy.
17. Decision making as to the type of intervention used in patients with unfavourable anatomy is still a matter of debate and must take into account the multifactorial nature of predicting the results of PMC.
TRICUSPID REGURGITATION
18. Relevant tricuspid regurgitation requires early intervention to avoid secondary damage of the RV.
19. Tricuspid regurgitation should be liberally treated at the time of left-sided valve surgery. Isolated surgery of severe secondary tricuspid regurgitation (with or without previous left-sided valve surgery) requires comprehensive assessment of the underlying disease, pulmonary haemodynamics, and RV function.
PROSTHETIC VALVES
20. The choice between a mechanical prosthesis and a bioprosthesis should be patient-centred and multifactorial based on patient characteristics, the indication for lifelong anticoagulation, the potential and risks of a re-intervention, and the informed patient preference.
21. Clinical assessment of prosthetic valves should be performed yearly and as soon as possible if new cardiac symptoms occur.
15 Gaps in evidence
Important gaps in evidence exist in the following aspects of VHD:
GENERAL COMMENTS
1. Prognostic value of CMR-derived indices in patients with aortic regurgitation, aortic stenosis, and mitral regurgitation.
2. Tools for risk stratification for the decision for intervention (including the avoidance of futile interventions) and the choice of the type of intervention (TAVI vs. SAVR for aortic stenosis, repair vs. replacement for mitral and aortic regurgitation).
3. In asymptomatic patients with aortic regurgitation, aortic stenosis, and mitral regurgitation, identification and evaluation of earlier markers of LV dysfunction (biomarkers, imaging, multimodality) as well as longitudinal and translational studies on progression.
4. Gender issues regarding pathophysiology, indications, and timing of treatment.
5. Minimum volumes of procedures that are required to achieve optimal results of intervention.
6. Safety and efficacy of NOACs in patients with surgical or transcatheter bioprostheses in the first 3 months after implantation.
7. Patient education for shared decision making and timely evaluation.
8. Systematic epidemiological data addressing the burden of rheumatic heart disease.
9. Advocacy of VHD.
AORTIC REGURGITATION
10. Potential differences in the risk of aortic complications depending on subtypes of aortic aneurysms (site and morphology), as well as in patients with bicuspid aortic valves.
11. Further evaluation of surgical aortic valve repair.
AORTIC STENOSIS
12. Pathophysiology of progression and novel therapeutic targets for medical treatment.
13. Further research to evaluate the role of intervention:
• a. Long-term durability of transcatheter heart valves in comparison with surgical bioprostheses.
• b. Role of intervention (SAVR or TAVI) in asymptomatic patients.
• c. Role of TAVI in younger low-risk patients, patients with aortic stenosis affecting bicuspid valves, and patients with moderate aortic stenosis and LV impairment.
• d. Results of re-intervention (valve or coronary) after TAVI or SAVR.
• e. The role of revascularization in patients with severe aortic stenosis and asymptomatic concomitant CAD.
MITRAL REGURGITATION
14. Association between PMR and sudden cardiac death and ventricular arrhythmias.
15. Role of genetic testing to mitral valve prolapse.
16. Further evaluation of the role of intervention:
• a. Long-term results of transcatheter intervention.
• b. Indications of transcatheter intervention in patients with severe PMR at lower surgical risk.
• c. Potential impact of mitral valve intervention (surgery and catheter intervention) on survival in patients with SMR.
• d. Selection of criteria to identify responders to TEER for SMR (severity criteria, concept of ‘disproportionate mitral regurgitation’).
• e. The role of newer transcatheter treatment options (annuloplasty, combined repair techniques, valve replacement).
MITRAL STENOSIS
17. Scores predicting the results and complications of PMC, particularly that of severe mitral regurgitation.
18. Role of transcatheter mitral valve implantation in high-risk patients, particularly in patients with severe degenerative mitral stenosis and MAC.
TRICUSPID REGURGITATION
19. Quantification of tricuspid regurgitation severity and evaluation of RV function.
20. Further research to evaluate the role of intervention:
• a. Criteria for optimal timing of surgery in primary tricuspid regurgitation.
• b. Evidence on the clinical impact, timing, and treatment modality of isolated severe secondary tricuspid regurgitation.
• c. Criteria for concomitant tricuspid valve surgery at the time of left-sided surgery in patients without severe tricuspid regurgitation.
• d. Results and indications of transcatheter tricuspid valve treatment.
COMBINED AND MULTI-VALVE DISEASES
21. Further evaluation of the impact on outcomes and modalities of transcatheter intervention to better define the indications for intervention.
PREGNANCY
22. Optimal management of pregnant women with MHVs regarding antithrombotic regimens.
NON-CARDIAC SURGERY
23. Evaluation of the role of ‘urgent TAVI’ in the management of patients with severe aortic stenosis undergoing NCS.
16 To Do and Not To Do


Supplementary data
To read the full content of this article, please download the PDF.

