
In recent years, the evaluation and management of patients with valvular heart disease (VHD) has changed dramatically and transcatheter therapies now offer a less invasive treatment for a wider range of patients with different diseases. In this revolution, a non-negligible role has been played by the advances in non-invasive imaging that currently allows an optimised evaluation of VHD. In addition, as well as preprocedural evaluation of the most suitable treatment strategy in each individual scenario, it permits the anticipation of potential technical issues and complications.
In this setting, the American College of Cardiology (ACC) and American Heart Association (AHA) have just released their updated guidelines on VHD management. Once again, they highlight the important priority of a careful evaluation of each single case by a multidisciplinary heart valve team (MDT), ahead of the need of defining the best treatment strategy for each patient. This definitely signals the beginning of a new era where transcatheter solutions flank a surgical approach for a considerable proportion of patients with VHD, independently from their risk profile.
Aortic valve
The prior ACC/AHA focus update on VHD management in 2017 was released a few days before the publication of the SURTAVI randomised clinical trial (RCT) on intermediate-risk severe aortic stenosis (AS) patients. Therefore, it did not incorporate key evidence regarding AS treatment, which resulted in its being already outdated when published. As a consequence, it was expected that the 2020 ACC/AHA guidelines would have signalled a considerable jump forward for transcatheter aortic valve implantation (TAVI) indications (Figure 1).
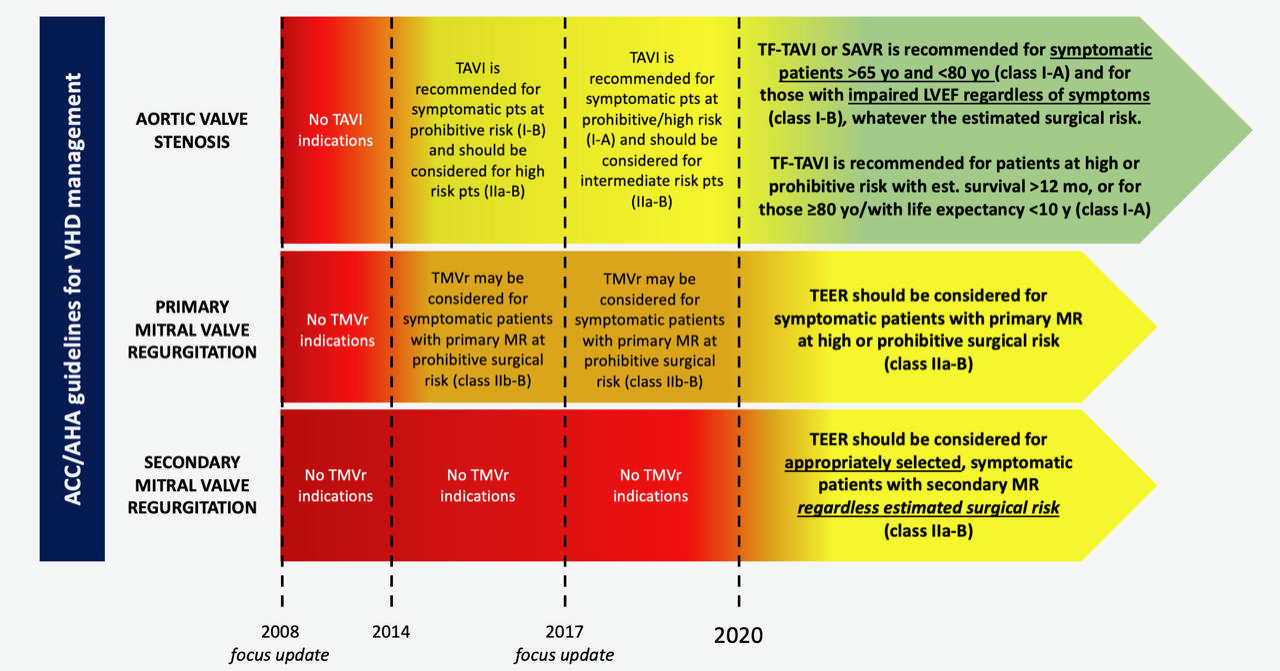
Figure 1. Updates of American College of Cardiology/American Heart Association (ACC/AHA) indications for transcatheter valve interventions. LVEF: left ventricle ejection fraction; MR: mitral regurgitation; SAVR: surgical aortic valve replacement; TEER: transcatheter edge-to-edge repair; TF-TAVI: transfemoral transcatheter aortic valve implantation; TMVr: transcatheter mitral valve repair
In fact, these latest guidelines have made a remarkable paradigm shift in the selection of AS patients, valuing age and anticipated life expectancy more than risk profile itself; in other words, the guidelines convey that TAVI in patients at low surgical risk should not be considered a taboo anymore. In detail, the class of recommendation of either transfemoral (TF) TAVI or surgical aortic valve replacement (SAVR) has been equalised for symptomatic patients aged 65-80 years (Class I, level of evidence [LOE] A) and for those who are still asymptomatic with an impaired left ventricle ejection fraction (LVEF) (Class I, LOE B), regardless of the preprocedural estimated risk. Furthermore, among patients in whom a bioprosthesis is appropriate, TF-TAVI should be preferred among patients of any age with high/prohibitive risk, if predicted survival after intervention is >12 months with an acceptable quality of life, and in patients >80 years of age or with life expectancy <10 years (Class I, LOE A)1(Figure 1).
The threshold of 65 years for TAVI has probably exceeded the expectations of the community considering the available evidence, but the task force has given a strong emphasis to life expectancy to avoid an inappropriate use of TAVI in younger patients. In particular, even if transcatheter aortic valve (TAV) durability data are increasing, to date some surgical bioprostheses have evidence of a longer durability and patients included in the RCTs were mostly older than 70 years. Indeed, it is mandatory to evaluate the best therapy for each patient properly, taking into account life expectancy, valve durability and other long-term considerations. In this connection, it is important to consider the possibility of a future need of repeating the intervention for bioprosthesis degeneration.
Of note, the recommendations for repeat intervention have not changed: SAVR should be preferred to TAVI in case of low-to-intermediate surgical risk. This seems to clash with the updated recommendation for treatment of native aortic valve stenosis, as redo SAVR has a considerably higher risk per se. Indeed, TAVI has already been demonstrated to have sustained clinical and haemodynamic outcomes up to three years, even in the setting of transcatheter valve-in-valve (ViV) implantation for degenerated surgical aortic bioprostheses (TAV-in-SAV)2. Nevertheless, although repeat TAVI for TAV degeneration (TAV-in-TAV) has been shown to be feasible and to have a higher procedural success rate compared to TAV-in-SAV3, it has to be highlighted that important issues regarding coronary access and sinus of Valsalva sequestration have recently been raised. Therefore, it is mandatory to consider this aspect during the selection of the first TAVI procedure. In fact, the use of a first TAV that extends its frame to the ascending aorta and has a supra-annular structure would represent a relevant issue for ensuring future coronary access after TAV-in-TAV in particular anatomical scenarios4.
Finally, TAVI is now indicated as an alternative to SAVR even for treatment of isolated, severe bicuspid aortic valve stenosis (Class IIb, LOE B); however, a proper assessment of patient-specific procedural risks, as well as considerations about the expected outcomes with TAVI, has to be carefully considered in the decision-making process1.
Mitral valve
A significant step forward has also been made regarding the indications for transcatheter treatment of severe mitral regurgitation (MR).
It should be noted that, to date, the expansion in MR treatment recommendations has been somewhat difficult due to the complexity of the mitral valve apparatus and the heterogeneity of causes underlying this valvular disease.
Notwithstanding this, the results from the MITRA-FR and COAPT RCTs in 2018 have allowed the ACC/AHA guidelines to expand the recommendations for transcatheter edge-to-edge repair (TEER) to secondary MR treatment5,6. Furthermore, for the first time, these trials have permitted defining accurate echocardiographic parameters that identify patients who could benefit significantly in terms of procedural outcomes as well as in prognosis. In particular, patients with “disproportionate” MR (large effective regurgitant orifice area [EROA] in the presence of not severely enlarged left ventricular volumes) have been shown to benefit from TEER in terms of prognosis compared to guideline-directed medical therapy. As a result, patients with isolated secondary MR should be considered for TEER regardless of surgical risk estimation, after a careful selection by echocardiographic specialists with expertise in mitral valve transcatheter treatment (Class IIa, LOE B)1(Figure 1).
In addition to this, TEER has obtained the same class of recommendation even for patients with severe primary MR who are considered at high or prohibitive surgical risk, when durable outcomes are expected with the transcatheter treatment.
Mitral transcatheter treatment is an ever-changing field. Further transcatheter solutions are currently under investigation for approval and are expected to be available soon in clinical practice. In particular, increasing effort has focused on transcatheter mitral valve replacement (TMVR) during recent years. Indeed, several manufacturers have been developing their own devices for TMVR, as surgery data have demonstrated that chordal sparing mitral valve replacement has better procedural outcomes at midterm compared to valve repair. Therefore, TMVR promises to transform MR treatment indications in the near future.
The 2020 ACC/AHA recommendations for VHD management have signalled an important step in the adoption of less invasive transcatheter therapies, especially for AS and MR treatment. Considerations regarding the age and life expectancy of patients are crucial aspects to take into account during MDT evaluation, as a considerable proportion of patients are suitable for a transcatheter approach and can benefit from it independently from their risk profile.
Conflict of interest statement
M. Barbanti is a consultant for Edwards Lifesciences and an advisory board member for Medtronic. G. Costa has no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

