
Introduction
Percutaneous valve technologies have revolutionised the treatment of valvular heart disease (VHD) and emerged as an alternative treatment option in elderly patients at all levels of surgical risk. In 2019 and 2020, important advances in the field of valvular heart interventions were achieved. In this review, we present the most recent and relevant studies that have emerged on the transcatheter treatment of VHD in 2019 and 2020.
AORTIC STENOSIS
In August 2019, the U.S. Food and Drug Administration (FDA) approved the use of transcatheter aortic valve replacement (TAVR) for the treatment of symptomatic severe aortic stenosis (AS) in low-risk patients. This approval formalised the application of TAVR in patients at all levels of surgical risk.
TAVR FOR LOW-RISK PATIENTS
The aforementioned expanded FDA indication for TAVR was based on the results from the PARTNER 3 and Evolut Low Risk trials.
The PARTNER 3 trial was an independently evaluated, non-inferiority, randomised controlled trial (RCT) comparing outcomes between TAVR with the Edwards SAPIEN 3 balloon-expandable valve (BEV; Edwards Lifesciences, Irvine, CA, USA) system and surgical aortic valve replacement (SAVR) among symptomatic AS patients with Society of Thoracic Surgeons Predicted Risk of Mortality (STS PROM) score <4%1. The primary endpoint (a composite of all-cause death, stroke, or rehospitalisation) at one year was lower in the TAVR group than in the surgery group (8.5% vs 15.1%; p=0.001 for superiority). The trial demonstrated that patients at low surgical risk treated with a balloon-expandable (BE) system experienced good short-term clinical outcomes with the rates of death or disabling stroke at one year being 1.0%. Indeed, these data concerning the SAPIEN 3 BEV serve to demonstrate superiority of a transcatheter heart valve (THV) system over surgery. The two-year results were reported at the American College of Cardiology (ACC) 2020 Scientific Session. At two years, 11.5% of TAVR patients had attained the composite outcomes, compared with 17.4% of SAVR patients (hazard ratio [HR] 0.63, 95% confidence interval [CI]: 0.44-0.88; p=0.007). No difference in stroke was observed (TAVR vs SAVR: 2.4% vs 3.6%, HR 0.68, 95% CI: 0.31-1.40; p=0.28). Differences were sustained for rehospitalisation (TAVR vs SAVR: 8.5% vs 12.5%; HR 0.67, 95% CI: 0.45-1.00; p=0.046). At two years, moderate paravalvular leak (PVL) was present in 0.5% of TAVR patients and none of the surgery patients. Mild PVL was present in 26.0% of TAVR patients and 2.3% of surgery patients.
The Evolut Low Risk trial was a multicentre, randomised non-inferiority trial, which evaluated a self-expanding (SE) supra-annular THV, including three valve generations (CoreValve®, Evolut™ R/PRO; all Medtronic, Minneapolis, MN, USA) in 1,403 low surgical risk AS patients (STS PROM <3%)2. The 24-month incidence of the primary endpoint (a composite of all-cause death or disabling stroke) was 5.3% in the TAVR group and 6.7% in the surgery group. TAVR showed non-inferiority to surgery (difference, −1.4 percentage points; 95% Bayesian credible interval for difference, −4.9 to 2.1; posterior probability of non-inferiority >0.999).
The approved use of TAVR in low-risk patients paves the way for expansion of TAVR into patient groups that were traditionally the remit of the surgeon. However, both low-risk trials must be interpreted within the context of the patient population studied and several questions remain regarding the short-term nature of these data3. Compared to SAVR, TAVR with the SAPIEN 3 and Evolut devices had similar or higher rates for PVL, new permanent pacemaker implantation (PPM) and new left bundle branch block (LBBB). TAVR with the SAPIEN 3 had a comparable rate of moderate/severe PVL with SAVR at one year in the PARTNER 3 trial (0.6% vs 0.5%) but a higher rate of new LBBB at one year (23.7% vs 8.0%). TAVR with the Evolut device had a higher rate of moderate/severe PVL (3.6% vs 0.6%) and more frequent in-hospital new LBBB (24.1% vs 11.7%) as compared with SAVR in the Evolut Low Risk trial. At one year, a higher rate of new PPM with the Evolut was observed in the Evolut Low Risk trial (TAVR vs SAVR: 19.4% vs 6.7%), while TAVR with the SAPIEN 3 had a comparable PPM rate with SAVR (TAVR vs SAVR: 7.3% vs 5.4%). Whether these outcomes have long-term adverse effects in low-risk patients is uncertain, given the fact that they have been associated with increased mortality or prolonged hospitalisation in previous studies4,5,6. Future follow-up data of both studies will help to determine the long-term impact of new LBBB, PPM and PVL in this younger patient population. Importantly, high-risk anatomy (e.g., annular calcification, unfavourable coronary anatomy) and bicuspid aortic valves (BAV) were excluded from both trials (20.3% in PARTNER 3, 12.5% in Evolut Low Risk), making the conclusions not generalisable to the overall AS population, preserving a role for SAVR in these patients with challenging anatomies. More importantly, long-term THV durability and difficulties for future coronary access remain unclear in a potentially younger subset of patients. The results from these two trials show us that, in the short term, TAVR seems comparable or even superior to SAVR, but longer-term evaluation of outcomes specifically in the context of the clinical implications of PVL, LBBB and valve durability are needed. The results of PARTNER 3 and Evolut Low Risk are shown in Table 1.
Recently, Kolte et al performed a meta-analysis which included 2,887 patients from four RCTs comparing TAVR versus SAVR in low-risk patients7. Within the TAVR arm, 66.9% of patients received a self-expanding valve (SEV). Mean STS PROM was 2.3%. Compared with SAVR, TAVR was associated with lower risk of all-cause death (2.1% vs 3.5%; relative risk [RR] 0.61, 95% CI: 0.39-0.96; p=0.03) and cardiovascular death (1.6% vs 2.9%; RR 0.55, 95% CI: 0.33-0.90; p=0.02) at one year. TAVR and SAVR had similar rates of stroke (3.0% vs 4.2%; RR 0.68, 95% CI: 0.43-1.07; p=0.10), myocardial infarction (1.7% vs 2.1%; RR 0.78, 95% CI: 0.46-1.34; p=0.37), or valve/heart failure (HF) rehospitalisation (5.2% vs 7.9%; RR 0.72, 95% CI: 0.42-1.23; p=0.23), and major vascular complications (3.6% vs 2.4%; RR 1.66, 95% CI: 0.89-3.11; p=0.11). TAVR patients had lower rates of new/worsening atrial fibrillation (10.0% vs 39.4%; RR 0.27, 95% CI: 0.20-0.32; p<0.001), life-threatening/disabling bleeding (3.9% vs 11.2%; RR 0.37, 95% CI: 0.24-0.55; p<0.001), and acute kidney injury stage 2/3 (0.7% vs 2.9%; RR 0.26, 95% CI: 0.13-0.52; p<0.001), but were more likely to need PPM (17.4% vs 5.5%; RR 3.85, 95% CI: 1.73-8.58; p=0.001) and have moderate/severe PVL (3.6% vs 1.7%; RR 2.16, 95% CI: 1.03-4.54; p=0.04). Data from this meta-analysis suggest that TAVR is associated with lower all-cause and cardiovascular related mortality compared to SAVR at one year in low-risk patients with severe AS. Another meta-analysis by Siontis et al included seven trials which randomly assigned 8,020 participants to TAVR (4,014 patients) and SAVR (4,006 patients)8. The authors found that, compared with SAVR, TAVR is associated with reduction in all-cause mortality and stroke up to two years irrespective of baseline surgical risk and type of THV system. Data from these two meta-analyses suggest that, compared to SAVR, TAVR has a favourable outcome up to two years in patients with severe AS regardless of baseline surgical risk.
Current evidence might bring TAVR into guideline recommendations for low-risk patients with severe AS who are candidates for bioprosthetic aortic valve replacement and have suitable anatomy for TAVR. However, the uncertainty about the long-term impact of PPM and PVL and the lack of evidence on long-term durability and the feasibility for future coronary access need to be ascertained before a class A recommendation is given to TAVR by the guidelines.
TAVR VERSUS SAVR IN A BROAD, REAL-WORLD PATIENT POPULATION
In contrast to selective populations in randomised TAVR trials, the UK TAVI trial involved a broad group of patients who were treated at every medical centre that performs TAVR across the United Kingdom. Presented at ACC 2020, the primary outcome (one-year all-cause mortality) was comparable between TAVR and SAVR (4.6% vs 6.6%, p=0.23). TAVR was associated with less bleeding and shorter hospital stay, but more vascular complications, PPM, and PVL. Stroke rates were similar between treatment groups.
The UK TAVI trial confirms the effectiveness of the TAVR strategy in a real-world setting. However, adequate long-term follow-up of real-world data, albeit difficult to achieve, is needed to confirm sustained clinical benefit and valve durability.
TAVR FOR ASYMPTOMATIC PATIENTS WITH SEVERE AS
Current European guidelines recommend surgery for asymptomatic patients with severe AS with left ventricular dysfunction9. It remains unclear whether patients with severe asymptomatic AS and normal left ventricular ejection fraction (LVEF) will benefit from surgery. In the RECOVERY trial10, 145 patients with asymptomatic severe AS were randomised to SAVR versus “watchful waiting”. A primary endpoint (a composite of operative mortality or cardiovascular death during the entire follow-up) event occurred in one patient (1%) versus 11 patients (15%) (HR 0.09, 95% CI: 0.01-0.67) and all-cause death in 7% of patients versus 21% of patients in SAVR versus “watchful waiting” (HR 0.33, 95% CI: 0.12-0.90), respectively. The cumulative incidence of sudden death was 4% at 4 years and 14% at 8 years in the “watchful waiting” group. These results showed improved long-term (8 years) survival among patients with asymptomatic but very severe AS with normal LVEF treated with SAVR compared with the “watchful waiting” strategy. The RECOVERY trial has several limitations including selective use of exercise testing, which is important to rule out symptoms. It included a relatively small sample of young patients with more frequent BAV disease, few coexisting morbid conditions, and low operative risk. Moreover, crossover occurred in 5% of patients in the early surgery group and in 3% of patients in the “watchful waiting” group. Additionally, this trial was not blinded; the non-fatal outcomes could have been influenced by the clinician’s knowledge of the treatment strategy10. Waiting for symptoms to emerge before intervening with SAVR in asymptomatic patients with severe AS may increase the risk of death. Although there is probably an acceptable waiting period for this population, exactly how long that period is and which parameters should be followed remain uncertain. Currently, enrolment of patients with asymptomatic but severe AS in the EARLY TAVR (NCT03042104) trial is ongoing, randomising patients to either TAVR or clinical surveillance. The study intends to enrol 1,109 patients. The primary endpoint is a non-hierarchical composite of all-cause death, stroke, and unplanned cardiovascular hospitalisation. December 2021 is estimated to be the primary completion date. EARLY TAVR might help to define “adequate” watchful waiting, so that patients could benefit from early intervention before symptoms occur.
TRIALS COMPARING DIFFERENT TAVR DEVICES
As TAVR emerges as a treatment option for patients with severe symptomatic AS for all surgical risk groups, trials comparing different TAVR devices are needed to demonstrate the individual strengths and limitations of each device. This would improve device selection for an individual patient. The SCOPE I trial was a prospective, non-inferiority study of 739 patients comparing the safety and efficacy of the ACURATE neo™ (Boston Scientific, Marlborough, MA, USA) to the SAPIEN 3 THV among low to high surgical risk patients (mean STS PROM 3.5%; 8% high risk, 55% intermediate risk)11. The primary endpoint (a combination of VARC-2 safety and clinical efficacy criteria at 30 days) occurred in 87 (24%) patients in the ACURATE neo and in 60 (16%) patients in the SAPIEN 3 group; non-inferiority of the ACURATE neo was not met (absolute risk difference 7.1% [upper 95% confidence limit 12.0%], p=0.42). Secondary analysis showed a significantly increased incidence of the primary endpoint at 30 days in the ACURATE neo group compared with SAPIEN 3 (95% CI for risk difference: −1.3 to −12.9, p=0.02). The incidence of all-cause death (2% vs 1%) and stroke (2% vs 3%) was similar between groups, but the risk of acute kidney injury (3% vs 1%) and moderate/severe prosthetic aortic regurgitation (AR) (9% vs 3%) were more common in the ACURATE neo group, compared to the SAPIEN 3 cohort. In contrast, the ACURATE neo valve had lower transvalvular gradients and a larger effective orifice area (EOA) post procedure. Longer-term outcomes are eagerly awaited.
The PORTICO IDE trial was a multicentre, non-inferiority evaluation of the safety and effectiveness of the SE Portico™ TAVR system (Abbott Vascular, Santa Clara, CA, USA) compared with FDA-approved and commercially available TAVR systems (SAPIEN, SAPIEN XT/3 [all Edwards Lifesciences], CoreValve, Evolut R/PRO) among 750 high and extreme risk patients. The study met both the pre-specified primary safety composite endpoint (all-cause mortality, disabling stroke, life-threatening bleeding requiring blood transfusion, acute kidney injury requiring dialysis, or major vascular complications at 30 days, 13.8% vs 9.6%; p-non-inferiority=0.034) and the primary effectiveness composite endpoint (all-cause mortality or disabling stroke at one year, 14.8% vs 13.4%, p-non-inferiority=0.006). At two years, rates of death (22.3% vs 20.2%, p=0.40) or disabling stroke (3.1% vs 5.0%, p=0.23) were similar between groups12. The results indicate that TAVR with the Portico system met criteria for non-inferiority for safety and efficacy compared with other commercially available THVs. However, the pacemaker insertion rate (27.7% vs 11.6%) and moderate/severe PVL (6.3% vs 2.1%) were higher with Portico.
Of note, the SE ACURATE neo valve was inferior to the BE SAPIEN 3 in the SCOPE I trial, while the SE Portico system was non-inferior for safety and efficacy compared with the group of BE (SAPIEN, SAPIEN XT/3) and SE (CoreValve, Evolut R/PRO) THV systems in the PORTICO IDE trial. The variances in results could mainly be attributed to the different components of the primary endpoints in these two trials. In the PORTICO IDE trial, the primary endpoint did not include PVL, which occurred infrequently in a BEV such as the SAPIEN 3 valve.
SOLVE-TAVI is a multicentre, randomised trial of 447 patients with AS undergoing TAVR comparing SEV (Evolut R) with BEV (SAPIEN 3). At 30 days, the primary composite endpoint of all-cause mortality, stroke, moderate or severe PVL, and PPM was equivalent between SEV and BEV (28.4% vs 26.1%, p-equivalence=0.04). Event rates for the individual components were as follows: all-cause mortality 3.2% versus 2.3% (p-equivalence <0.001), stroke 0.5% versus 4.7% (p-equivalence=0.003), moderate/severe PVL 3.4% versus 1.5% (p-equivalence=0.0001), and PPM 23.0% versus 19.2% (p-equivalence=0.06) in SEV versus BEV patients13.
Interestingly, the SOLVE-TAVI study included PPM and PVL in the composite primary endpoints, which is different from mainstream published data. In addition, PPM rates among BEV are relatively higher than published data14.
Recently, the five-year outcomes of the CHOICE trial were published15. There were no statistically significant differences between BEV and SEV in all-cause death (53.4% vs 47.6%, p=0.38), cardiovascular death (31.6% vs 21.5%, p=0.12), all strokes (17.5% vs 16.5%, p=0.73), and repeat HF hospitalisation (28.9% vs 22.5%, p=0.75). Forward flow haemodynamics were significantly better with the SEV. Moderate or severe structural valve deterioration (SVD) was uncommon but occurred more frequently with the BEV. Since half of the study population is dead at five years, any inference to other outcome data is limited by the immortal time bias. Furthermore, the study is limited in power, and limited in that the devices used are now no longer current generation, and results between BE and SE THVs may differ as the devices evolve.
TAVR FOR PATIENTS WITH BICUSPID AORTIC VALVE STENOSIS
Studies comparing SAVR to TAVR have systematically excluded AS patients with bicuspid anatomy. Concern for poor clinical and/or procedural outcomes in this patient subset stem from anatomical and technical challenges. These patients are typically younger, have a high incidence of concomitant aortopathy, and their degenerated aortic valves often have severe calcification. All these features increase the risk of valve embolisation, aortic root injury, stroke, badly expanded frame, and, potentially, reduced durability. However, a previous retrospective study showed that TAVR in BAV was feasible with encouraging short- and intermediate-term clinical outcomes16. The safety and efficacy of TAVR in BAV in its various phenotypes is currently the subject of several ongoing clinical evaluations.
Makkar et al evaluated the results of 2,691 propensity-matched pairs of patients with BAV versus tricuspid aortic valve (TAV) stenosis undergoing implantation of the SAPIEN 3. There was no difference in 30-day mortality (BAV vs TAV: 2.6% vs 2.5%, p=0.82) or one-year mortality (BAV vs TAV: 10.5% vs 12.0%, p=0.31). However, the 30-day risk of stroke was significantly greater among those with BAV (BAV vs TAV: 2.5% vs 1.6%, p=0.02)17.
The BIVOLUTX trial is a prospective, multicentre registry of 151 patients undergoing TAVR with the Evolut R/PRO for BAV stenosis. Results were reported during the PCR e-Course 2020. Device success was observed in 96% of the patients. At 30 days, all-cause mortality occurred in 3.3% of the cohort, but only 1.9% were cardiovascular deaths. The rates of disabling stroke and major vascular complications were 3.3% and 4.6%, respectively. The incidence of PPM was 19.6%. The 30-day mean EOA was 2.1 cm2, and the mean gradient was only 7.3 mmHg. Only 2% of patients had moderate regurgitation, and none had severe regurgitation. Patient-prosthesis mismatch was observed in 10.5% of the cohort, but only 1.3% was severe.
The Evolut Low Risk Bicuspid Study prospectively tracked 150 TAVR patients at 25 centres in the USA. The results were presented at ACC 2020. At 30 days, the primary safety endpoint (death or disabling stroke) occurred in 1.3% of patients. The device success rate was 95.3%, and 99.3% of patients survived the procedure, 96% showed correct positioning of the valve and 100% had mild or no AR. These findings show that the SEV works well for patients with BAV, though additional follow-up is necessary to determine long-term outcomes. Recently, a propensity matching analysis of the STS/ACC TVT Registry (929 matched pairs) showed that the rates of all-cause mortality at 30 days (2.6% vs 1.7%, p=0.18) and 1 year (10.4% vs 12.1%, p=0.63), as well as the rate of stroke at 30 days (3.4% vs 2.7%, p=0.41) and 1 year (3.9% vs 4.4%, p=0.93), were comparable between BAV and TAV stenosis. In patients at increased surgical risk, TAVR with SEV for BAV stenosis showed acceptable safety outcomes with low complication rates18.
The BEAT registry included 353 consecutive patients who underwent TAVR using new-generation Evolut R/PRO or SAPIEN 3 valves in BAV. After propensity score matching, device success was similar between the groups (SAPIEN 3 = 85.7% vs Evolut R/PRO = 84.4%, p=0.821). At one year, the rates of overall death and cardiovascular death were similar between the groups. The study confirms the feasibility of both SAPIEN 3 and Evolut R/PRO implantation in BAV anatomy; a higher rate of moderate-severe PVL was observed in the Evolut R/PRO group at one year in the matched cohort, although patients treated with BEV had a higher rate of annular rupture19.
Patients with BAV frequently have an excellent outcome with SAVR. Current data on TAVR in BAV are encouraging but as yet limited. Therefore, before extending TAVR technology to lower-risk patients with BAV, further data are needed to elucidate device-host interaction, optimal sizing strategy, and the need for a cerebral protection device considering the relatively higher stroke rates in TAVR for BAV.
TAVR FOR PATIENTS WITH LARGE OR SMALL AORTIC ANNULI
Large or small aortic annuli are two other relevant anatomic features that could influence the haemodynamic and clinical outcomes after TAVR. Early experiences in TAVR for these two anatomic features show encouraging results. The 29 mm SAPIEN 3 valve is recommended for annular areas ranging between 338 and 683 mm2. Recently, Sengupta et al reported the one-year outcomes of TAVR in extremely large annuli with the SAPIEN 3, including 105 patients across 15 centres with a mean area of 721.3±36.1 mm2 (683.5 to 852.0 mm2) who underwent TAVR20. Procedural success was obtained in all patients; there was no annular rupture, embolisation, or coronary obstruction. One-year mortality and stroke rates were 18.2% and 2.4%, respectively. Mild PVL occurred in 21.7% of patients, while moderate/greater PVL occurred in 4.3%. Mild and moderate/severe transvalvular AR occurred in 11.6% and 0%, respectively. Valve gradients remained stable at one year. These results show that SAPIEN 3 TAVR in annular areas >683 mm2 is feasible, with favourable midterm outcomes.
A small aortic annulus and a small aortic root have been associated with increased ischaemic cardiovascular events and mortality21. In 2019, Freitas-Ferraz et al reviewed the clinical challenges and current therapeutic alternatives for the treatment of AS in patients with a small aortic annulus22. TAVI-SMALL is a retrospective registry of patients with severe AS and small annuli (annular perimeter <72 mm or area <400 mm2 on computed tomography) treated with SEVs (Evolut R, n=397; Evolut PRO, n=84; ACURATE, n=201; Portico, n=177)23. No significant differences were reported in terms of severe prosthesis-patient mismatch (overall rate 9.4%; p=0.134), PPM (15.6%), and periprocedural and one-year adverse events23. These results suggest that transcatheter SEVs have optimal clinical results in patients with small annuli. Currently, there is no dedicated randomised trial comparing TAVR versus SAVR in patients with small aortic annuli. The VIVA trial (NCT03383445), a prospective randomised trial planning to enrol 300 patients, is ongoing to compare the valve haemodynamic performance (incidence of severe prosthesis-patient mismatch and moderate/severe AR) between TAVR and SAVR in severe AS patients with small aortic annuli. This study will be the first head-to-head clinical trial to provide randomised data comparing TAVR and SAVR in patients with severe AS and small aortic annuli.
THE DURABILITY OF BIOPROSTHETIC VALVES
As TAVR is extended to low-risk patients, younger patients will probably be treated and, by extension, there will be a greater proportion of patients with longer life expectancy. Hence, the durability of transcatheter technology will become a very important topic for future studies. Although the true durability of surgical bioprostheses is unclear, only limited medium-term and no long-term data describe THV durability.
Follow-up from the NOTION trial and the UK TAVI registry suggests that SVD and failure rates in THVs beyond five years remain low. In the analysis of the NOTION trial, moderate/severe SVD was defined as a mean gradient ≥20 mmHg, an increase in mean gradient ≥10 mmHg from three months post procedure, or more than mild intraprosthetic AR either new or worsening from three months post procedure24. Bioprosthetic valve failure (BVF) was defined as valve-related death, aortic valve reintervention, or severe haemodynamic SVD. Despite similar all-cause mortality between SEV and SAVR at six years (42.5% and 37.7%, respectively, p=0.58), moderate/severe SVD occurred in 24% of SAVR patients compared with only 4.8% of TAVR patients (p<0.001). These disparate rates relate largely to inferior haemodynamics achieved with the surgical prostheses rather than more rapid leaflet deterioration. Indeed, BVF was low and similar for both TAVR and SAVR groups up to six-year follow-up (6.7% vs 7.5%, p=0.89). Definite endocarditis (5.9% vs 5.8%, p=0.95) and non-structural valve deterioration (NSVD, moderate/severe patient-prosthesis mismatch or moderate/severe PVL at three months) (57.8% vs 54.0%, p=0.52) were not different between groups and there was no clinical valve thrombosis in either group. The results from the NOTION trial are encouraging for the durability of THVs; however, some limitations should be acknowledged, including the use of now outdated THV technology and the use of echocardiography- rather than CT-based valve sizing. In the surgical group, an algorithm to avoid patient-prosthesis mismatch was not mandated. Finally, the echocardiographic assessment of SVD was not adjudicated by a core laboratory.
The UK TAVI registry is a prospective mandatory database that includes all patients undergoing TAVR in the United Kingdom25. The incidence of moderate SVD (the definition of haemodynamic SVD was adapted from the 2017 EAPCI/ESC/EACTS criteria) was 8.7% (mean 6.1 years post implantation) – 57% due to new AR and 43% due to restenosis. Sixty-two percent (62%) of patients with moderate SVD had received an SEV, while 38% had received a BEV. There were no cases of NSVD. Severe SVD occurred in just one patient at 5.3 years after implantation with an SEV26.
Although these data are encouraging for TAVR durability, longer-term studies are warranted before TAVR is routinely extended to patients with longer life expectancy. Some lessons from the surgical experience should be heeded in this respect. 1) Even poorly designed surgical valves (e.g., the Mitroflow A2; Sorin Group, Saluggia, Italy) did not demonstrate accelerated SVD until eight years post implantation. 2) Small changes in valve design, as has been seen with THV technology since the NOTION and UK TAVI data were initially collected, can have a considerable impact on durability.
With this latter lesson in mind, the results of the five-year follow-up from the PARTNER 2A trial may be particularly relevant27. These data showed no significant difference in the risk of death or disabling stroke between BEV TAVR and SAVR (47.9% and 43.4%, respectively; HR 1.09, 95% CI: 0.95-1.25; p=0.21) in intermediate-risk patients. At five years, more patients in the TAVR group than in the surgery group had at least mild PVL (33.3% vs 6.3%). Repeat hospitalisations were more frequent after TAVR than after surgery (33.3% vs 25.2%), as were aortic valve reinterventions (3.2% vs 0.8%)27. Moreover, an analysis of SVD in the PARTNER 2A trial, presented at PCR London Valves 2019, found that the SAPIEN 3 had similar durability to surgical valves, with the second-generation SAPIEN XT demonstrating lower midterm durability than surgery. Of course, this now historical data set includes patients treated with echo-based rather than CT-based sizing (a recognised risk factor for several complications, including PVL), and the trial evaluated an earlier-generation THV device (SAPIEN XT) which has a higher rate of PVL compared to the current generation. Moreover, a sub-analysis starting at two years showed a higher risk of death/disabling stroke with TAVR both in the overall cohort (36.3% vs 29.5%; HR 1.27, 95% CI: 1.06-1.53) and in the transfemoral subgroup (34.0% vs 28.4%; HR 1.23, 95% CI: 1.00-1.52) compared with SAVR. Hence, results from long-term follow-up showing the durability of current-generation THVs are needed.
Recently, Sathananthan et al reported that the 10-year rate of SVD/BVF was low in patients who underwent TAVR with an early-generation BEV28. Of 235 patients who underwent TAVR between 2005 and 2009, 15 patients had SVD/BVF, with a cumulative incidence at 10 years of 6.5% (95% CI: 3.3%-9.6%). The rate of SVD/BVF at 4, 6, 8, and 10 years was 0.4%, 1.7%, 4.7%, and 6.5%, respectively. Nine patients had moderate SVD and six patients had severe SVD. Survivors (n=19) at 10-year follow-up, had a mean gradient of 14.0±7.6 mmHg and AR was ≥moderate in 5%28. The authors concluded that, using early-generation BEV in a high-risk population, there was a low rate of SVD/BVF at 10-year follow-up.
Despite 18 years of TAVR, data on durability are currently limited by the immortal time bias due to a high mortality rate among patients (mean age 84) included in early TAVR studies. Therefore, long-term data about TAVR in younger and low-risk patients might answer the durability question.
ANTITHROMBOTIC STRATEGY AFTER TAVR
Currently, it is not clear whether oral anticoagulation (OAC) should be considered after TAVR. On the one hand, OAC could reduce the risk of thromboembolic events after TAVR and has the potential to reduce the risk of leaflet thrombosis with TAVR; on the other hand, OAC increases bleeding and mortality in the elderly and frail TAVR population.
The results from the PARTNER 3 CT substudy demonstrated that hypo-attenuated leaflet thickening (HALT) was more frequent among transcatheter versus surgical valves at 30 days (13% vs 5%; p=0.03), but not at one year (28% vs 20%; p=0.19). Patients with HALT at both 30 days and one year, compared with those without HALT, had significantly increased aortic valve gradients at one year (17.8±2.2 mmHg vs 12.7±0.3 mmHg, p=0.04)29. The Evolut Low Risk LTI (leaflet thickening or immobility) substudy found that the presence of CT imaging abnormalities of aortic bioprostheses was frequent but dynamic in the first year after SE TAVR and SAVR; however, these findings did not correlate with aortic valve haemodynamics after aortic valve replacement in low-risk patients30. Hence, the routine use of OAC to avoid the development of leaflet thrombosis is not justified.
The GALILEO trial is a randomised, event-driven, multicentre study comparing a rivaroxaban-based strategy with a clopidogrel-based strategy after TAVR. Patients without established indication for OAC were randomised to either rivaroxaban 10 mg daily plus aspirin 75-100 mg or to clopidogrel 75 mg plus aspirin 75-100 mg after successful TAVR31. The trial was terminated prematurely because of safety concerns due to excess ischaemic and bleeding events in the rivaroxaban arm. After a median follow-up of 17 months, the primary efficacy outcome of death or first thromboembolic event occurred in 105 patients versus 78 patients in the rivaroxaban group and antiplatelet group, respectively (95% CI: 1.01-1.81, p=0.04). The primary safety outcome of major, disabling or life-threatening bleeding occurred in 46 and 31 patients, respectively (95% CI: 0.95-2.37, p=0.08). Importantly, all-cause mortality (7.7% vs 4.6%; HR 1.69, 95% CI: 1.13-2.53) and VARC major bleeding events (3.6% vs 1.8%; HR 2.02, 95% CI: 1.09-3.76) were significantly higher in the rivaroxaban group compared to the antiplatelet group. In the GALILEO-4D substudy, a lower incidence of HALT and hypo-attenuation affecting motion was observed in the rivaroxaban group three months after TAVR32. No significant differences in echocardiographic assessment, such as transvalvular gradients or PVL, between treatment strategies were reported.
The randomised POPular-TAVI trial assessed the safety of OAC alone compared to antiplatelet drugs alongside OAC for managing TAVR complication risks in patients with a long-term indication for OAC. The two primary outcomes were all bleeding and non–procedure-related bleeding at 12 months. Bleeding occurred in 21.7% of patients receiving OAC alone and in 34.6% of patients receiving OAC plus clopidogrel (RR 0.63, 95% CI: 0.43-0.90; p=0.01); most bleeding events were at the TAVR access site. Non–procedure-related bleeding occurred in 21.7% and in 34.0% of patients, respectively (RR 0.64, 95% CI: 0.44-0.92; p=0.02). In patients undergoing TAVR who were receiving OAC, the incidence of serious bleeding over a period of one month or one year was lower with OAC alone than with OAC plus clopidogrel33.
These findings have clear implications for future TAVR practice. 1) The routine performance of post-TAVR CT imaging for the detection of HALT or hypo-attenuation affecting motion is not justified. 2) The routine use of OAC post TAVR should not be considered in the absence of another indication. Future studies evaluating the dose reduction strategies of novel OAC regimens could have an impact on this latter recommendation.
TRANSCATHETER AORTIC VALVE DESIGN
Most THV systems are designed on either a BEV or a SEV concept (Lotus valve [Boston Scientific] is a mechanically expanding valve). It remains unclear, however, whether these different THV concepts achieve similar or different clinical outcomes. The randomised comparison of these two concepts was underpowered to detect differences in hard clinical outcomes between designs, but did demonstrate differences in valve haemodynamics, PVL and PPM34.
A propensity score-matched comparison of 7,820 patients undergoing TAVR based on the FRANCE-TAVI registry showed that the use of SEV was associated with a higher risk of PVL and/or in-hospital mortality, and two-year mortality compared with use of BEV35. The association of THV type (SEV) with two-year mortality remained after multivariable adjustment including PVL severity and other periprocedural events. This retrospective study cannot answer the question as to whether PVL is a cause of mortality or a marker for it, but it prompts us to look at it more closely, as other studies too have shown increased long-term mortality for moderate or severe PVL14,36.
This study highlights the need for RCTs sufficiently powered to compare head-to-head on individual efficacy endpoints for the different THVs, and the need to simplify and optimise the grading of PVL and investigate its long-term clinical impact. Also, this emphasises the importance of tailoring the choice of THV to each individual patient, which will need an adequate level of experience and volume for a given Heart Team.
QUANTITATIVE ASSESSMENT OF AR AFTER TAVR
As post-implantation regurgitation and PVL affect clinical outcomes, accurate and straightforward measurement of AR becomes an important means for determining the need for and effectiveness of various corrective interventions such as post-dilation or valve-in-valve. With the current minimalistic approach for TAVR, avoiding general anaesthesia, the use of intraprocedural transoesophageal or even transthoracic echocardiography (TTE) as a measurement tool for AR is limited.
Video-densitometry is a well-validated objective method that relies on aortography to quantify AR accurately and with great reproducibility37. Aortograms were analysed in vitro and clinically validated against magnetic resonance imaging38,39. Recently, the feasibility and reproducibility of this technique were tested in several real-world populations. In the RESPOND study, a prospective, single-arm study evaluating the outcomes following TAVR with the Lotus valve for patients with AS40, the quantitative assessment of AR using video-densitometry showed a good relationship with the core laboratory-adjudicated echocardiographic and visual angiographic findings, providing a more granular discrimination of regurgitation within the same strata of regurgitation assessed by echocardiography41. The multicentre ASSESS-REGURGE registry demonstrated high feasibility of the assessment of regurgitation with quantitative aortography and protocoled acquisition during TAVR procedures42. The OVAL (Online Video-densitometric Assessment of Aortic Regurgitation in the Cath-Lab) study, presented at PCR London Valves 2019, showed a high feasibility (92%) of online video-densitometric assessment immediately after THV deployment in the cath lab. Modolo et al performed a multicentre pooled analysis of 2,258 valves (seven different types) to assess the acute regurgitation following TAVR and to compare different implanted THVs using video-densitometry. They found that the Lotus valve, when compared to others, was the only one with less regurgitation after TAVR in the “real world”43. Taken together with previous data, these results may pave the way for the application of video-densitometry during TAVR in the cath lab. This online tool to quantify regurgitation during TAVR may facilitate decision making post implantation with respect to the requirement for post-dilatation, etc. Moreover, it may become a reproducible tool in future trials to quantify the sealing capacities of novel THV systems.
MITRAL REGURGITATION INTERVENTION
In 2018, the first randomised trials comparing transcatheter edge-to-edge mitral valve repair (TMVr) for secondary mitral regurgitation (MR) with the MitraClip® system (Abbott Vascular) against guideline-directed medical therapy (GDMT) alone were reported (MITRA-FR and COAPT)44,45,46. The latter showed significant improvement in mortality and HF hospitalisation, which was not proven in the former. Recently, the two-year follow-up results of the MITRA-FR study were published, and the three-year outcomes and important substudies of the COAPT trial were reported.
The two-year results of MITRA-FR only confirmed the initial results: at two years, all-cause death and unplanned HF hospitalisation occurred in 63.8% of patients in the intervention group and 67.1% in the control group (HR 1.01, 95% CI: 0.77-1.34). All-cause mortality occurred in 34.9% of patients in the intervention group and 34.2% in the control group (HR 1.02, 95% CI: 0.70-1.50). Unplanned HF hospitalisation occurred in 55.9% of patients in the intervention group and 61.8% in the control group (HR 0.97, 95% CI: 0.72-1.30)47. In patients with severe secondary MR, percutaneous repair added to GDMT did not significantly reduce the risk of death or HF hospitalisation at two years compared with GDMT alone. These results confirm that the difference between MITRA-FR and COAPT is rather due to differences in patient characteristics than to the length of follow-up, and should promote further research in the domain of percutaneous repair of secondary MR to identify better the ideal patients for these procedures.
The three-year outcomes of COAPT were presented at TCT 2019. In the intention-to-treat population, the benefit of TMVr persists at three years, in terms of HF hospitalisation, survival rate, functional capacity and quality of life, compared to GDMT. The primary endpoint of HF hospitalisation reached 81.5% for the GDMT group and 46.5% in the MitraClip group. The primary safety endpoint (freedom from device-related complications) was 8.7% at three years. All-cause death was 42.8% versus 55.5% (HR 0.67, 95% CI: 0.52-0.85, p=0.001) in MitraClip versus GDMT, respectively. The high mortality rates reflect the poor outcome of patients with advanced HF. Heart transplantation (HTx) remains the gold standard therapy for patients with advanced HF. Recently, the results of the MOMENTUM 3 trial showed that outcome following left ventricular assist device (LVAD) for patients with advanced HF could be similar to HTx48. It is unclear why the rates of LVAD use or HTx were low, 11.4% in the GDMT group and 7.3% in the MitraClip group in the COAPT trial.
The echocardiographic analysis49 from the COAPT trial has failed to pinpoint specific baseline echocardiographic factors that could help valve teams in deciding which patients with HF and severe or moderate-to-severe MR would benefit more from a MitraClip procedure. An inherent limitation is that within two years 46% and 29% of patients in the control and device groups, respectively, died, without available echo data and the authors used a multiple imputation method. The findings should reinforce the idea that, so long as patients now being selected for the procedure meet the criteria used in the COAPT clinical trial, the real-world population is likely to benefit to the same degree as patients in COAPT. Currently, there are no good echocardiographic criteria to identify who are the best responders and who might not respond to treatment of their MR. Further studies are warranted to determine echocardiographic predictors of outcome following MitraClip implantation.
The substudy of health status50 from the COAPT trial indicated that the health status benefit after edge-to-edge TMVr versus GDMT alone was consistent across all subgroups, except for patients with ischaemic cardiomyopathy, who derived greater health status benefit than those with non-ischaemic cardiomyopathy. At 24 months, 36.4% of edge-to-edge TMVr patients were alive with a moderately large (≥10-point) improvement versus 16.6% of standard care patients (p<0.001). In patients with HF and secondary MR, TMVr resulted in early, substantial and sustained improvement in health status while health status remained unchanged in the GDMT arm. These findings of improved symptoms and quality of life further support the use of the edge-to-edge technique of TMVr in patients with symptomatic HF due to 3+ to 4+ secondary MR. Moreover, among 551 patients in the COAPT trial with HF and secondary MR, improvement in short-term (baseline to one month) patient-reported disease-specific health status was independently associated with lower risk of subsequent death or HF hospitalisation between one month and two years (HR 0.86 per 10-point increase in the Kansas City Cardiomyopathy Questionnaire [KCCQ], 95% CI: 0.81-0.92, p<0.001). While patients treated with TMVr were far more likely to experience improvements in short-term health status, the association of health status changes with long-term outcomes did not differ by treatment assignment (p-interaction=0.17)51.
According to the patient-level economic analysis52 of the COAPT trial, in patients with HF and moderate-to-severe or severe secondary MR, edge-to-edge TMVr plus GDMT is a “reasonable” strategy, both clinically and economically. When the observed COAPT trial results were projected over a lifetime horizon, TMVr was associated with substantial gain in life expectancy and quality-adjusted life expectancy at an incremental cost of approximately US $45,000 per patient.
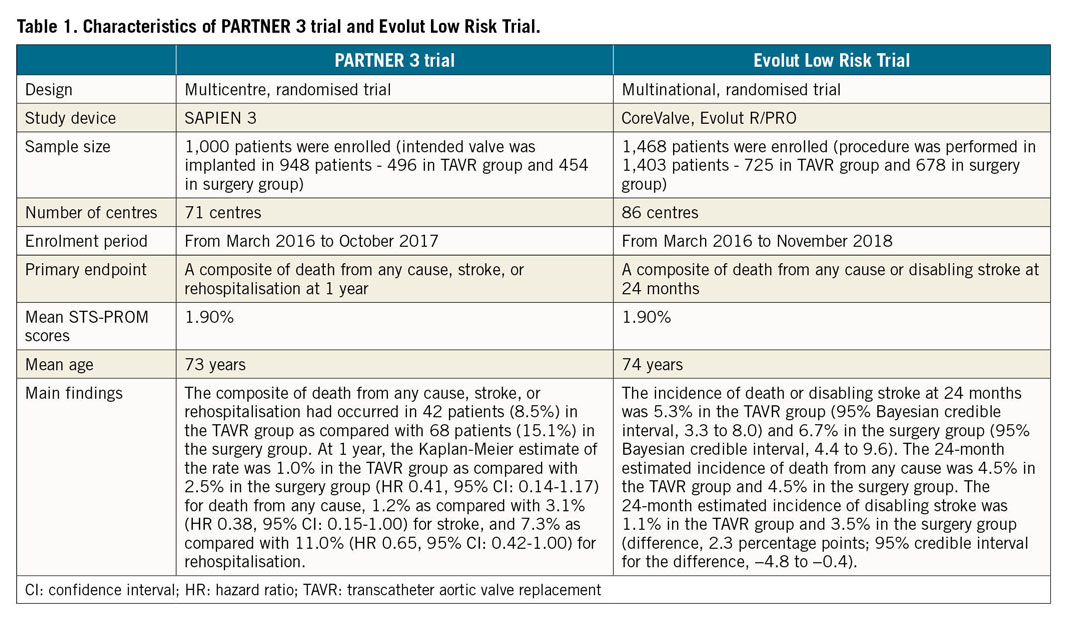
In March 2019, the FDA expanded the indication for MitraClip from symptomatic degenerative MR to secondary MR. It is the only FDA-approved repair device (Figure 1A). The next-generation MitraClip NTR and XTR device systems are safe and effective for patients with primary MR, according to results of the EXPAND trial, presented at ACC 2020. A total of 422 patients with symptomatic MR (≥3+) were included. The acute procedural success rate was 94.5%. The 30-day all-cause death rate was 2.4%. No patients experienced myocardial infarction, while 1.2% experienced stroke and 0.9% had a non-elective cardiovascular surgery to treat device-related complications. At 30 days, MR reduction to none/trace was achieved in 27.7% of primary MR patients, while MR ≤1+ was achieved in 86.9% and MR ≤2+ was achieved in 97.3% of patients.
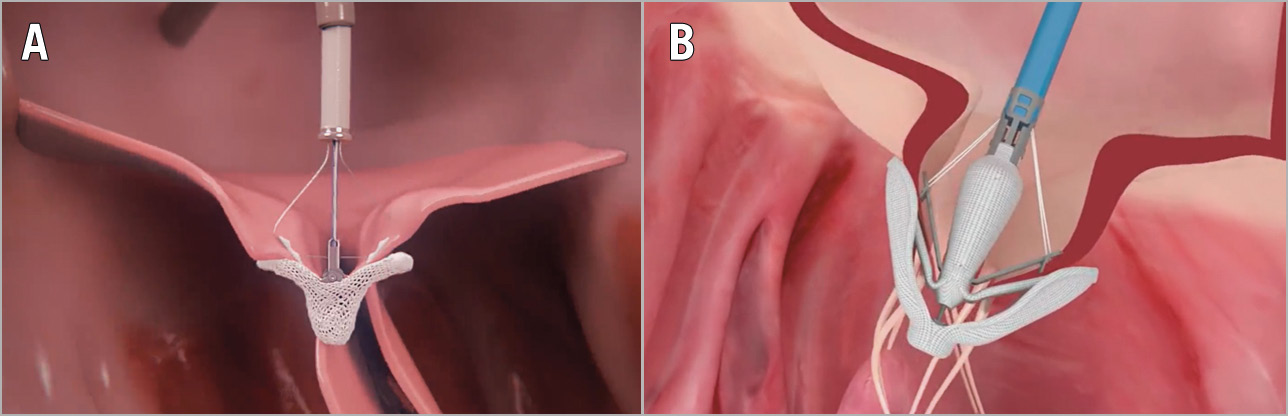
Figure 1. Transcatheter mitral valve repair devices. A) MitraClip Transcatheter Valve Repair System. B) PASCAL Transcatheter Valve Repair System.
The PASCAL transcatheter valve repair system (Edwards Lifesciences) is another edge-to-edge TMVr system for the treatment of an insufficient mitral valve through tissue approximation with an additional anatomic spacer between the two grasping paddles (Figure 1B). In February 2019, the PASCAL repair system received a CE mark for the treatment of patients with MR. The CLASP study is the first clinical study using the PASCAL system. It demonstrated feasibility and acceptable safety of the device among patients with severe MR on optimal medical therapy53. Patients had significant reduction in the severity of MR (86% had ≤1+ MR at 30 days) and notable functional improvement (85% in NYHA Class I/II). Major adverse event rates were low (6.5%) and the procedural success rate was 95%. At one year, Kaplan-Meier survival was 92% with 88% freedom from HF hospitalisation, MR was ≤1+ in 82% of patients and MR ≤2+ in 100% of patients, 88% were in NYHA Class I/II, and the KCCQ score improved 14 points (all p<0.001). The PASCAL system demonstrated a low complication rate and high survival, with robust sustained MR reduction accompanied by significant improvements in functional status and quality of life at one year. These results suggest that the PASCAL device may be an alternative option for percutaneous treatment of MR.
The Carillon® mitral contour system (Cardiac Dimensions, Kirkland, WA, USA), a mitral annuloplasty device, is designed to reduce the mitral annular dimension by virtue of the close anatomic relationship between the coronary sinus and the posterior mitral annulus. The REDUCE-FMR study aimed to evaluate the effects of the Carillon device on MR severity and left ventricular remodelling. The primary endpoint was change in mitral regurgitant volume at 12 months. The Carillon device reduced mitral regurgitant volume and left ventricular volumes significantly in symptomatic patients with functional MR receiving optimal medical therapy. Of note, in this study not all patients had echocardiograms of sufficient quality for MR quantification or left ventricular volume assessment, and fewer patients with moderate-severe FMR at baseline were enrolled54.
TRANSCATHETER MITRAL VALVE REPLACEMENT (TMVR) FOR MR
TMVr has been a potential option to reduce MR without the associated risks of surgery in selected patients. However, some patients remain suboptimal candidates for this treatment, and residual moderate or severe MR after leaflet repair has been reported in about 10% of real-world patients55,56. TMVR has emerged as a potential alternative treatment option in patients with severe MR that is anatomically unsuitable for edge-to-edge or other percutaneous repair systems.
Although prior studies have demonstrated the feasibility of TMVR, few studies have reported more than the feasibility and short-term safety with these devices. In 2019, the one-year results of patients in the initial feasibility study with the Tendyne device (Abbott Vascular, Roseville, MN, USA) were published57. This study suggests favourable early safety and effectiveness, with no intraprocedural deaths, no conversion to cardiac surgery, a low rate of major apical bleeding (1%), elimination of MR in 98.4% of patients treated, and symptom improvement in the majority (at one year, 88.5% of survivors were in NYHA Class I/II, compared with 34.0% at baseline, p<0.0001). One-year survival was 72.4%. The two-year results were presented at the PCR e-Course 2020. The two-year all-cause mortality was 41.6%. By two years, 93.2% still had no MR, with the remainder showing grade 1+ MR. Sustained clinical improvement was observed in terms of NYHA classification (81.7% in Class I/II at two years) and a 19-point improvement on the KCCQ (both p<0.0001). Moreover, HF hospitalisations fell from 1.30 to 0.51 per patient-year, p=0.01. This investigation confirms the potential for TMVR to treat high surgical risk patients with severe symptomatic primary and/or secondary MR effectively without the requirement for cardiopulmonary bypass, with a reasonable safety profile, and improved symptoms and quality of life in patients with HF. In January 2020, the Tendyne system received the world’s first CE mark for TMVR.
The first-in-human experience with the SAPIEN M3 (Edwards Lifesciences) system in high surgical risk patients with severe MR was published in 201958. During TCT 2019, the updated 30-day outcomes for the U.S. early feasibility study of the SAPIEN M3 TMVR system demonstrated technical feasibility, safety, and efficacy in reducing MR. The SAPIEN M3 TMVR system comprises a nitinol dock, which encircles the chordae tendineae, and a BE THV. The dock and THV form an ensemble, with the native mitral valve leaflets secured in between, thereby abolishing MR (Figure 2, Table 2). These early data suggest that application of this dock plus a modified SAPIEN 3 THV for the treatment of native MR warrants investigation in a large-scale trial.
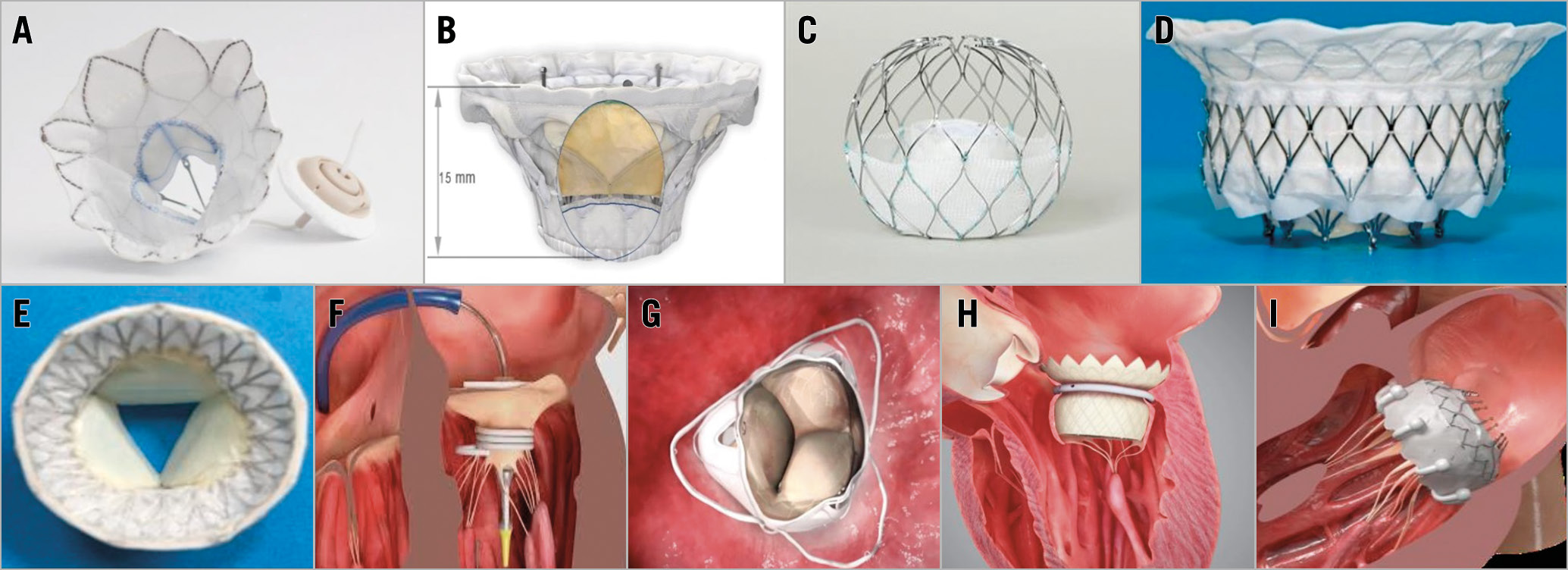
Figure 2. Transcatheter mitral valve replacement devices. A) Tendyne (Abbott). B) Cardiovalve (Valtech). C) AltaValve (4C Medical Technologies Inc). D) Intrepid (Medtronic). E) Cephea (Abbott). F) SAPIEN M3 (Edwards Lifesciences). G) Caisson (LivaNova). H) HighLife (HighLife SAS). I) EVOQUE (Edwards Lifesciences).
The results from the Edwards EVOQUE TMVR Early Feasibility Study were published in 2020. Technical success was achieved in 13 patients (92.9%) and one patient was converted to surgery. At 30 days, there was one non-cardiovascular death (7.1%), there were two strokes (14.3%), no myocardial infarctions, and no rehospitalisations. Two patients (14.3%) underwent PVL closure. One patient (7.1%) underwent alcohol septal ablation for left ventricular outflow tract obstruction. Including the two patients with PVL closure, MR was ≤mild in all implanted patients at 30 days with no MR in 10 (83.3%). Mean mitral gradient was 5.8 mmHg. Functional class improved to NYHA Class ≤II in nine patients (81.8%)59. This first-in-human experience has demonstrated the feasibility of the transseptal EVOQUE TMVR system.
During PCR London Valves 2019, the early experiences with several new TMVR devices were reported (Figure 2). Characteristics of the main TMVR devices are shown in Table 2. The early feasibility for TMVR with the Caisson transcatheter mitral valve (LivaNova, London, UK) was investigated in the INTERLUDE/PRELUDE studies. The results in successfully implanted patients have been encouraging: the procedure itself is feasible but technically challenging. However, LivaNova is ending the Caisson TMVR programme. The early feasibility study of the Cardiovalve (Valtech Cardio, Or Yehuda, Israel), which offers a transfemoral, transvenous delivery of the valve, is currently enrolling. A feasibility study of the HighLife™ transseptal transcatheter mitral valve (HighLife SAS, Paris, France) was also approved and started enrolling. Early procedural experience with the Cephea transseptal mitral valve system (Abbott, Abbott Park, IL, USA) was also presented. The results appear favourable with the early feasibility study currently in development. The AltaValve™ (4C Medical Technologies Inc., Maple Grove, MN, USA) is a TMVR device designed to broaden the treatable patient population: the device’s supra-annular fit and atrium-only fixation bypasses the concerns of anchoring and fixation difficulties present in current TMVR technologies. Moreover, AltaValve leaves the LV geometry intact and minimises the risks of left ventricular outflow tract obstruction and damage to the LV. The first-in-man transseptal experience of AltaValve was favourable and the early feasibility study is currently recruiting. These ongoing studies will provide more evidence on TMVR in the future. The results of MR intervention trials in 2019 and 2020 are summarised in Supplementary Table 1.
TRICUSPID REGURGITATION INTERVENTION
Tricuspid regurgitation (TR) is a common disease with poor clinical outcomes60. Previous studies indicate that both primary and secondary TR impact adversely on survival61,62,63. Recently, transcatheter tricuspid valve intervention (TTVI) has emerged as a potential therapeutic option for symptomatic severe/torrential TR in high surgical risk patients. The field of TTVI is in its infancy and there are currently no completed prospective or randomised controlled trials on this subject available. The fragile nature of TR patients and the not infrequent persistence of significant residual TR post TTVI confer considerable uncertainty regarding the real clinical efficacy of these techniques64,65.
The TriValve international registry is so far the first and largest multicentre, multi-device series of patients undergoing TTVI for severe TR66. A propensity-matched analysis from the TriValve registry67 suggested decreased all-cause mortality and reduced risk of death and HF hospitalisation in patients treated with a range of different TTVI systems for severe, symptomatic TR compared to medical therapy alone. A total of 472 patients were included in TriValve; after propensity score matching, there were 268 patients in each of the TTVI and GDMT arms. The primary endpoint was all-cause mortality or HF hospitalisation. At 12 months, compared to GDMT, TTVI patients had lower one-year mortality (23±3% vs 36±3%, p=0.001), rehospitalisation (26±3% vs 47±3%; p<0.0001), and a lower incidence of the composite endpoint (32±4% vs 49±3%; p=0.0003). This study is important as it provides the first evidence that transcatheter correction of TR is associated with improved clinical outcomes compared to GDMT. There are obvious limitations in this analysis, including the retrospective uncontrolled nature of both the transcatheter and medical intervention arms.
Percutaneous edge-to-edge repair with the MitraClip opens perspectives for patients with TR and increased surgical risk, according to new results from the prospective single-arm TRILUMINATE study68. One-year results from the TRILUMINATE study were shared at the PCR e-Course 2020. At one year, data showed a TR reduction of at least one grade in 87.1% of patients, a low all-cause mortality rate (7.1%), consistent signs of reverse right ventricular remodelling, and improvements in quality of life. In April 2020, the TriClip™ (Abbott) received its CE mark: it is the first minimally invasive, clip-based tricuspid valve repair device to be commercially available in the world.
The TRI-REPAIR study evaluated the safety and performance of TR repair with the Cardioband transcatheter tricuspid valve reconstruction system (Edwards Lifesciences) in a single-arm, multicentre trial of 30 inoperable patients with moderate to severe functional TR. Six-month outcomes show that the system performs as intended and appears to be safe in patients with symptomatic and moderate to severe functional TR69. The one-year results of patients with functional TR, a technically challenging group, showed an efficient and effective reduction in annular size and regurgitant orifice area with the Cardioband system. There is a high survival rate at one year (83.3%). Moreover, Edwards received the CE mark for the Cardioband system for the treatment of TR in 2018. The system is the first commercially available transcatheter therapy for the treatment of TR. The ongoing post-market TriBAND study (NCT03779490) may provide more evidence for this system.
FORMA is a coaptation tricuspid valve repair device (Edwards Lifesciences) designed to increase the leaflet coaptation surface by occupying the regurgitant orifice area. Short-term and midterm results have been reported previously70,71. In 2019, the long-term outcomes from the first-in-human experience of the FORMA were published72. At 32 months, less than severe TR was observed on echocardiography in 67% of patients. Compared with baseline, significant improvements in NYHA class (p<0.001), six-minute walk test (+54 m, p=0.016) and KCCQ score (+16 points, p=0.016) were found in 15 patients at follow-up of at least 24 months. The FORMA system showed a favourable long-term safety profile in high-risk patients, with sustained functional improvement and acceptable TR reduction up to three years. However, the FORMA programme has been discontinued by Edwards.
The first-in-human experience of the PASCAL repair system for severe TR showed that procedural success was 86%; no intraprocedural complications occurred. At 30 days, mortality was 7.1%, and 88% of patients were in NYHA Class I/II, with TR grade ≤2+ in 85%. Six-minute walk distance improved from 240 to 335 m (p<0.001)73. This first-in-human experience evaluating transcatheter tricuspid repair with the PASCAL system demonstrated high procedural success, acceptable safety, and significant clinical improvement. In May 2020, the PASCAL transcatheter valve repair system received its CE mark for the treatment of TR.
Little is known about intermediate-term valve-related outcomes after transcatheter tricuspid valve-in-valve or valve-in-ring replacement. In 2019, important data from the VIVID registry were published74. A total of 306 patients were followed over a median duration of 15.9 months after transcatheter tricuspid valve replacement (TTVR). The cumulative three-year incidence of death, reintervention, and valve-related adverse outcomes was 17%, 12%, and 8%, respectively. There was a low rate of tricuspid THV reintervention (31/306), endocarditis (8/306), and leaflet thrombosis (8/306) after TTVR in patients with prior TV replacement or repair. During the PCR e-Course 2020, another analysis of the VIVID registry was reported: 1,079 patients from 90 centres were included, median follow-up was 492 days. The four-year Kaplan-Meier survival rate was 62.5% in valve-in-valve versus 49.5% for valve-in-ring replacement (p<0.001). Mitral valve-in-ring patients required more redo mitral valve replacement at four years. Residual MR was associated with higher mortality. Residual mitral stenosis was not predictive of patient mortality but was associated with repeat mitral valve replacement. Suboptimal haemodynamics of mitral valve-in-valve and mitral valve-in-ring should lead to procedural strategies to improve post-implantation haemodynamics in order to optimise device durability. The results of TR intervention trials are summarised in Supplementary Table 2.
Clinical implementation of transcatheter tricuspid valve replacement is still in its infancy. Existing dedicated TTVR devices (NaviGate [NaviGate Cardiac Structures, Inc., Lake Forest, CA, USA], TricValve [P & F Products and Features, Vertriebs GmbH, Vienna, Austria], Trisol [Trisol Medical, Yokneam, Israel], Lux [Jenscare Biotechnology, Ningbo, China], TRiCares [TRiCares, Paris, France]) only have limited experience for the treatment of TR. Nevertheless, numerous new devices under development or at different stages of investigation will shed light on this field and provide some evidence on early safety and feasibility of TTVR.
Furthermore, dedicated iterative devices for TR continue to emerge. While small single-arm studies are expected to provide feasibility and early safety information, larger-scale randomised trials will follow in due course.
Conclusions
VHD remains a major societal and economic burden worldwide. Various new and improved transcutaneous treatment technologies for the treatment of VHD have emerged in 2019 and 2020. In addition, some important landmark trials were published. These data highlight the improved device technologies, procedural techniques and advances in imaging, resulting in positive data on the safety and feasibility of many transcatheter devices for treating patients with aortic, mitral and tricuspid valvular disease. These positive clinical trial results are associated with expanding indications and an increase in the number of patients who can now potentially be treated with transcatheter valvular therapy. The field of valvular heart interventions is growing extensively not only in terms of technological evolutions of the devices and procedural techniques, but also with innovations and improved experience in the field of cardiovascular imaging and resultant adaptations during the Heart Team decision-making process. The new decade looks promising for valvular heart interventions.
Guest Editor
This paper was guest edited by Alec Vahanian, MD, PhD; Department of Cardiology, Hôpital Bichat-Claude Bernard, and University Paris VII, Paris, France.
Conflict of interest statement
P.W. Serruys reports personal fees from Biosensors, Sino Medical Sciences Technology, Philips/Volcano, Xeltis, and HeartFlow, outside the submitted work. D. Mylotte reports being a proctor and consultant for Medtronic and MicroPort, being a consultant for Biosensors and Boston Scientific, and having received institutional research grants from Medtronic and Biosensors. R. Modolo reports research grants from SMT and Biosensors outside the work. O. Soliman reports research grants and core lab activities from several industry-sponsored clinical trials for which he receives no direct compensation. The other authors have no conflicts of interest to declare. The Guest Editor is a consultant for Edwards Lifesciences.
Supplementary data
To read the full content of this article, please download the PDF.

