
Surgical treatment remains the cornerstone of valvular heart disease (VHD) treatment1. Surgery was the only option for all types of VHD until 1984 when the first report of transseptal percutaneous mitral balloon commissurotomy (PMC) in mitral rheumatic disease was published2,3. Shortly after followed the first report of balloon aortic valvuloplasty by Cribier and colleagues4. Some time later, first-in-man reports were successively published on transcatheter aortic valve implantation in 2002 and mitral edge-to-edge repair in 2004, followed more recently by mitral bioprosthetic replacement and tricuspid repair5,6,7,8. Nowadays, PMC is an established routine treatment for rheumatic mitral stenosis (more than 20 years of experience)9. With the publication earlier this year of the PARTNER 3 and Evolut Low Risk trials, transcatheter aortic valve implantation (TAVI) was validated as an alternative to open heart surgery for the treatment of all risk patients and is en route to becoming a first-line therapy in most patients with severe aortic stenosis10.
Having observed the success story of TAVI, clinicians, researchers and the device industry are currently showing incredible dynamism to develop transcatheter solutions for mitral and tricuspid valves. More than 20 different devices exist or are in development and competition is fierce11,12,13. However, higher anatomical and pathophysiological challenges as well as the underestimated impact of mitral regurgitation (MR) and tricuspid regurgitation (TR) on clinical outcomes have slowed down the development of effective transcatheter solutions.
Given the wide range of information available and the potential for individual physician biases, team-based care has great potential merit. The role of cardiac surgeons in the multidisciplinary Heart Team is to offer a balanced and complementary approach to patient care by joint and shared decision making among the different medical care stakeholders. By exploring the multiple options available and sharing them with patients and their families where applicable, more optimal shared decision making is achieved, along with a tailored recommendation for therapy for a more informed and engaged patient.
The surgeon has the role of optimisng this patient-centric care which requires that the patient and family be sufficiently educated about the alternatives available so that their expectations can be met as fully as possible.
Is there still a place for open heart surgery for the treatment of VHD?
SURGICAL INDICATIONS
Ascending aorta surgery, infective endocarditis, severe congenital pathology, and complications of transcatheter procedures are likely to remain surgical indications. A rheumatismal condition often yields multiple valve disease or complex single mitral disease in young patients, which are conditions more prone to surgery, be it mitral repair or replacement. While PMC is applicable to rheumatic mitral stenosis with favourable anatomy, mitral regurgitation is an indication for surgery. Although promising, reports of combined transcatheter procedures remain scarce14,15,16. As of now, open surgical correction of several coexisting valvulopathies yields the most durable results. Valve replacement with mechanical prostheses in very young patients with severe rheumatic or congenital valve disease yields a life-long risk of valve thrombosis and major bleeding due to anticoagulation with vitamin K antagonists. In the future, this population could benefit from advances in transcatheter valve-in-valve and valve-in-ring technologies, if affordable, which would allow many very young patients to avoid the life-long risks of mechanical valves.
Conditions eligible for both transcatheter and surgical options: the place of the Heart Team
For patients potentially eligible for established transcatheter or surgical treatments, a trade-off between invasiveness and feasibility can be observed for treatment options of VHD. Because of its direct access to the diseased valve, open heart surgery has few anatomical constraints regarding the feasibility of correction. On the other hand, transcatheter-delivered devices and their delivery systems are less invasive. However, they are often designed to be compatible with the anatomy of most patients, but not all. Some patients who are theoretically eligible for transcatheter therapies present anatomical constraints incompatible with existing devices and their delivery systems. These constraints are related to fixed device sizes, local anatomy (e.g., unicuspid or bicuspid aortic valve for TAVI, insufficient coaptation length or short leaflets for MitraClip® [Abbott Vascular, Santa Clara, CA, USA]), and vascular approaches (e.g., transfemoral for TAVI, transseptal for MitraClip).
Aortic stenosis
Regarding aortic stenosis, although data are reassuring for extra-thoracic alternative TAVI pathways (transsubclavian, transcarotid), their non-inferiority to the transfemoral approach remains to be validated17. Both the PARTNER 3 and Evolut Low Risk trials excluded patients with bicuspid (and unicuspid) aortic valves. Bicuspid anatomy is often associated with ascending aorta aneurysm; both conditions can be optimally managed by open heart surgery. Concurrent existence of severe coronary artery disease with an indication for interventional treatment poses the question whether patients should be treated with percutaneous coronary intervention (PCI) before, during or after TAVI, or undergo surgical aortic valve replacement (SAVR) with coronary artery bypass grafting (CABG). Furthermore, data from randomised trials and meta-analyses confirm a twofold to fourfold increased risk of the need for a permanent cardiac pacemaker due to postoperative conduction abnormalities, and chronic cardiac stimulation may lead to secondary left ventricle dysfunction and heart failure18,19,20.
Heart Team discussion is likely to be the most appropriate solution to dilemmas posed by complex issues regarding indications for TAVI versus SAVR. Low-risk patients with aortic stenosis who could rather benefit from SAVR are those with (or who are):
– Bicuspid or unicuspid valves.
– Associated valvulopathy or indication for CABG: SAVR provides the possibility to associate several interventions through the same pathway, with reported durable results.
– Small roots with low coronary ostia take-off at risk of coronary obstruction.
– No iliofemoral pathway possible: despite important progress regarding TAVI pathways, some patients are ineligible for the iliofemoral route21.
– No or extreme calcification: the THV needs to be deployed in a solid frame to be anchored in the aortic annulus.
– High risk of pacemaker, notably with pre-existing LV dysfunction: a risk of long-term left ventricle dysfunction induced by cardiac stimulation has been reported18,22.
– Asymptomatic: the use of TAVI has never been evaluated in this condition. A dedicated randomised controlled trial (RCT) (EARLY TAVR [NCT03042104]) is on the way (Figure 1).
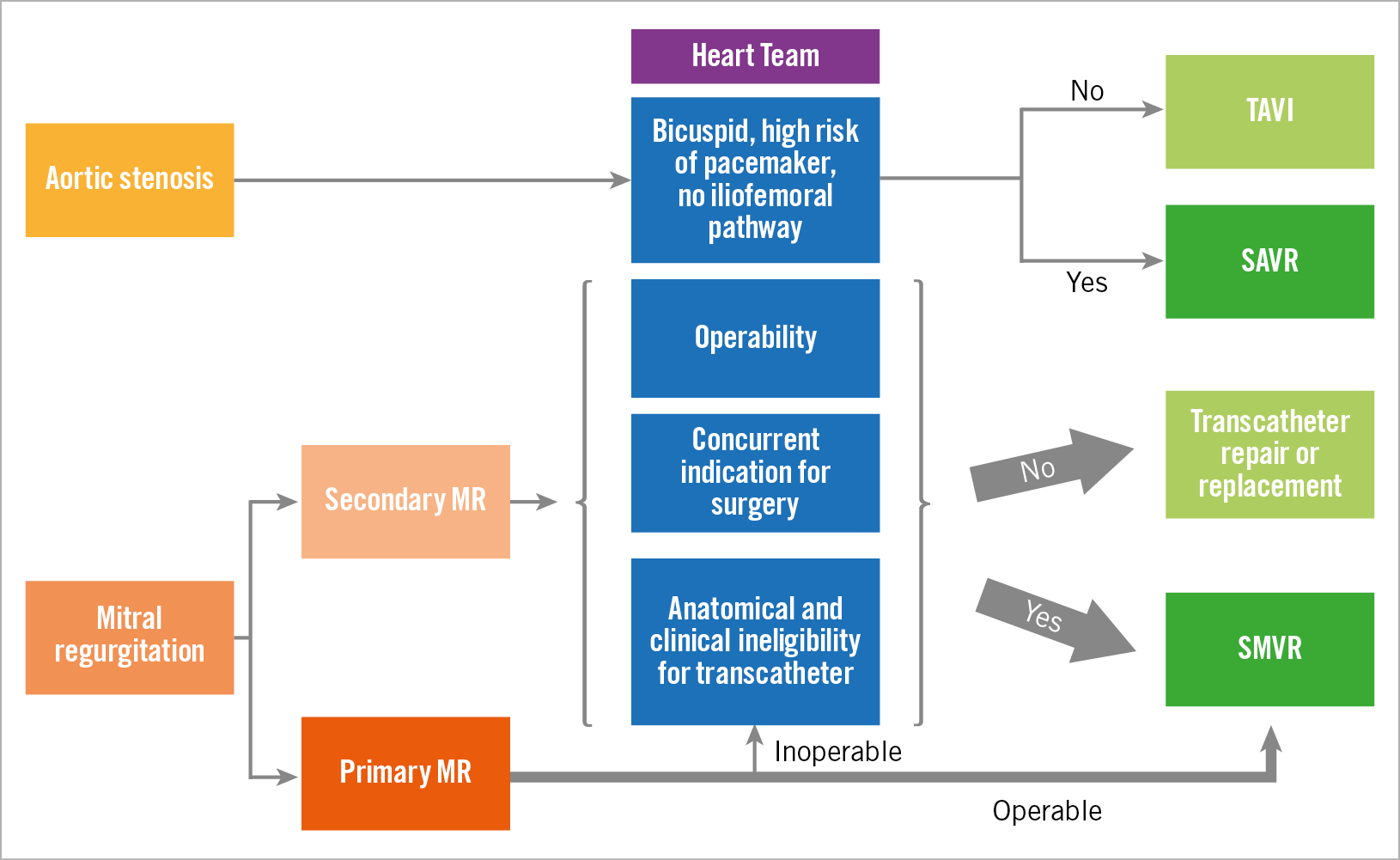
Figure 1. Proposed approaches for severe aortic stenosis and mitral regurgitation (central illustration). MR: mitral regurgitation; TAVI: transcatheter aortic valve implantation; SAVR: surgical aortic valve replacement; SMVR: surgical mitral valve replacement
Aortic regurgitation
As far as aortic regurgitation is concerned, most patients are treated with surgery, and TAVI remains at a preliminary stage23. Dedicated transcatheter heart valve designs may improve results in this challenging patient subset. Transfemoral TAVI for aortic regurgitation is at an early stage of evaluation24. Besides, ascending aorta aneurysm and/or aortic annulus dilatation are frequently associated with aortic regurgitation and, given the absence of transcatheter solutions for an ascending aortic aneurysm, its presence might limit indications for TAVI for aortic regurgitation to inoperable patients.
Mitral stenosis
Mitral stenosis of rheumatic origin is most frequently treated by percutaneous mitral commissurotomy. However, unfavourable anatomy such as extensive leaflet calcification, leaflet mobility reduction, very severe leaflet and subvalvular thickening would see surgical valve replacement being chosen as first-line therapy25. These elements have long been validated as being associated with worse outcomes of the percutaneous treatment and favour surgery, even more so if the clinical characteristics are also unfavourable. We currently have few resources to treat mitral valve regurgitation and/or stenosis associated with severe degenerative mitro-annular calcification (MAC) in inoperable and high-risk patients. Valve-in-MAC using TAVI devices amenable transseptally yielded 30-day mortality rates as high as 35% in the initial experience26. However, optimised screening using echocardiography and multislice computed tomography (MSCT) in candidates has been advocated to be the key to improving outcomes and eligibility. Appropriate candidates could be patients with circumferential calcification and a large left ventricle outflow tract (LVOT), which might reduce the risk of embolisation, LVOT obstruction and periprocedural mortality27. The solution might also come from valve-in-MAC transcatheter mitral replacement using THVs designed for the mitral valve, but further research is warranted28.
Mitral regurgitation
Severe secondary MR can be treated surgically when there is an indication for concomitant CABG. Secondary MR in high-risk patients with severe MR, anatomy compatible with the MitraClip, and without excessive left ventricle dilatation (left ventricle end-systolic diameter <70 mm) and dysfunction (left ventricle ejection fraction <20%) can be treated with the MitraClip with an expected clinical benefit29,30. The MitraClip device currently enjoys a comfortable advance on other devices designed to treat mitral regurgitation with several published randomised trials showing its feasibility and benefits on mortality (hazard ratio [HR] 0.62, 95% confidence interval [CI]: 0.46–0.82; p<0.001 for mortality) and heart failure rehospitalisations (HR 0.53; 95% CI: 0.40–0.70; p<0.001 rehospitalisation for heart failure) in selected patients with a disproportionately severe functional MR29,30,31,32. In patients without an indication for CABG, anatomy incompatible with the MitraClip, and no major comorbidities, Heart Team discussion is appropriate to decide upon the best strategy among mitral valve surgery (mitral valve and subvalvular apparatus repair or replacement), heart transplantation, mechanical circulatory support (left ventricle assistance device), optimal medical therapy or palliative care.
Validation of MitraClip use in primary MR needs further research33,34. The EVEREST II trial data argued towards lack of efficacy of the MitraClip, with high rates of residual and recurrent significant MR, but MitraClip use was associated with less procedure-related morbidity than surgery. However, since publication of the EVEREST II trial in 2011, several iterations of the MitraClip device have been developed and operators have gained experience with the device. Whether this progress can be translated into clinical benefit remains to be proven in dedicated studies. However, some anatomical incompatibilities are likely to remain for primary MR, including severe Barlow’s disease, calcification, and rheumatic mitral disease.
Combining transcatheter repair techniques has been proposed to mitigate the lack of efficacy concerning MR resolution; however, this poses the question of increased complexity and cost35,36. Transcatheter mitral valve replacement could provide a solution. Surgery, however, remains the most suitable option in most patients. Furthermore, the surgical community tends to work on reducing the invasiveness of mitral procedures. As such, robotic and mini-invasive thoracotomy or endoscopic surgery reduce scars and recovery time. However, the feasibility of this type of access is often conditioned by simple anatomy37.
Tricuspid valve
The tricuspid valve pathology in adults is essentially represented by tricuspid regurgitation (TR) secondary to left ventricle dysfunction. Evidence is limited for isolated tricuspid valve interventions. Surgery is currently the gold standard therapy for TR in the form of annuloplasty, leaflet repair or valve replacement, and associated with other cardiac interventions such as left-sided heart valve surgery or CABG25. Nevertheless, transcatheter tricuspid valve interventions could follow a similar path to TAVI. The first step would be to provide evidence of improvement in symptoms or survival in inoperable and high-risk patients as compared to medical treatment and surgery. Preliminary registry data suggest that transcatheter edge-to-edge repair is feasible. Data on annuloplasty and valve replacement are rare. However, clinical benefit and reproducibility remain to be proven in large studies with follow-up8.
Patient with previous valve surgery
Bioprosthesis degeneration has plagued bioprosthetic surgical replacement. Aortic bioprostheses could have an expected 15-year lifespan (degeneration rates at 15 years are between 15 and 35% depending on the make of the device)38,39. The interventional community addressed this issue with evidence suggesting feasibility and efficacy of valve-in-valve TAVI for degenerated aortic bioprostheses in patients ineligible for redo SAVR. Regarding mitral bioprosthetic degeneration, 30-day mortality rates of valve-in-valve with TAVI devices have been reported to be between 4 and 6% and provide a possible alternative to medical therapy in inoperable patients26,40. Valve-in-ring yielded a 30-day mortality rate of 10% in published series but results could be improved by better patient screening favouring interaction of the new THV and the pre-existing ring, avoiding large, rigid, oval or incomplete rings in order to reduce the risk of embolisation, high residual gradient, paravalvular leak and LVOT obstruction27. Similarly to the mitral valve, tricuspid bioprosthesis degeneration or annuloplasty ring failure has reportedly been treated with valve-in-valve and valve-in-ring transcatheter replacement treatment using TAVI with the SAPIEN (Edwards Lifesciences, Irvine, CA, USA) or the Melody™ (Medtronic, Minneapolis, MN, USA) pulmonary valve devices. However, patient selection remains difficult, and complications such as embolisation, malpositioning and paravalvular leak are frequent after valve-in-ring procedures41,42.
Overall, it is reasonable to expect a reduction of indications for redo SAVR or redo surgical mitral valve replacement for aortic and mitral bioprosthesis degeneration, respectively. With better patient screening, valve-in-ring procedures could progressively supplant redo mitral surgery for failure of annuloplasty rings in the future. There is also hope that valve-in-valve interventions could provide a valuable solution for tricuspid bioprosthesis degeneration. However, further development of transcatheter devices adapted to interventions for failing tricuspid rings is required.
Conclusion
Transcatheter interventions and surgery offer complementary solutions for optimal care in patients with valvular heart disease. The Euro Heart survey conducted at the beginning of the century showed that VHD was undertreated43. Recent registry data show that the advent of transcatheter technologies such as TAVI and the MitraClip, as well as progress in surgery, allow more patients to benefit from VHD correction44.
As evidence accumulates for transcatheter treatment of isolated severe aortic stenosis, surgery currently remains the cornerstone of the treatment for most patients with primary mitral regurgitation and tricuspid regurgitation. However, the future looks bright for transcatheter valve intervention. There is a need to improve and validate transcatheter valve devices in comparative trials and studies with prolonged follow-up. Furthermore, patient selection and procedure planning can be improved by multimodality imaging. As research advances faster than guideline iterations, Heart Team discussion is the main guarantor that every patient is offered the optimal solution for her/him based on contemporary evidence-based argumentation.
Finally, device deployment on a broad scale will require a balance to be achieved between potential public health improvement of patients with valvular heart disease, and financial constraints in high-income European countries and North America. It is important to remember that financial constraints often restrict interventional options solely to surgery in low-income countries. Developing and deploying transcatheter devices that are cheaper to make and implant remains a global unmet need for VHD that needs to be addressed.
Conflict of interest statement
T. Modine is a consultant for Boston Scientific, Medtronic, Edwards, Cephea, MicroPort, GE, and Abbott, and has received a research support grant from Edwards. A. Vahanian is a consultant for CardioValve. The other author has no conflicts of interest to declare.

