
Abstract
Aims: Despite rising rates of cardiogenic shock (CS), data on trends and in-hospital outcomes of short-term non-durable mechanical circulatory support (MCS) are limited. Thus, we aimed to identify recent national trends in MCS utilisation in the USA, patient-level predictors of MCS use, and in-hospital outcomes in CS inclusive of extracorporeal membrane oxygenation (ECMO).
Methods and results: Hospitalisations of US adults with a discharge diagnosis of CS, from January 2004 to December 2014, in the National Inpatient Sample were included. Rates of MCS were stratified by device type and clinical presentation. Outcomes included in-hospital mortality, hospitalisation costs, and number of procedures. A total of 183,516 hospitalisations with CS (47,636 [25.9%] involving MCS) were included. MCS recipients were younger, less frequently female, received more procedures, had higher costs, and more frequently presented with MI (MCS vs. non-MCS: 71.6% vs. 42.9%; p<0.0001). Growth in CS hospitalisations (214.4%) outpaced annual MCS use (160.0%), with relative declines in intra-aortic balloon pump use starting in 2008. Right heart catheterisation rates for both groups remained low (MCS vs. non-MCS: 5.9% vs. 3.3%; p<0.0001). In-hospital mortality declined but remained high in both groups (MCS vs. non-MCS [2014]: 32.7% vs. 41.5%; p<0.0001).
Conclusions: In-hospital mortality for CS has declined but remains high. Rates of CS have outpaced MCS utilisation which remains uncommon in non-MI hospitalisations with shock. MCS is associated with utilisation of other procedures during hospitalisation.
Abbreviations
CS: cardiogenic shock
ECMO: extracorporeal membrane oxygenation
IABP: intra-aortic balloon pump
ICD-9-CM: International Classification of Diseases, Ninth Revision, Clinical Modification
MCS: mechanical circulatory support
MI: myocardial infarction
ND-MCS: non-durable mechanical circulatory support
NSTEMI: non-ST-elevation myocardial infarction
PCPS: percutaneous cardiopulmonary support
STEMI: ST-elevation myocardial infarction
Introduction
Cardiogenic shock (CS), the inability to maintain adequate perfusion due to failure of the heart’s pumping mechanism, is present in 3.5% of patients presenting with acute heart failure and in 6.6% of those presenting with myocardial infarction (MI), with in-hospital mortality rates as high as 70%1,2. The recent availability of temporary mechanical circulatory support (MCS) has led to a redefinition of therapeutic options for CS, allowing circulatory support as a bridge to recovery or transplantation3. While durable MCS devices such as left ventricular assist devices and the total artificial heart are utilised selectively, the recent availability and rapid expansion of use of percutaneous short-term MCS devices, such as Impella® (Abiomed, Danvers, MA, USA) devices and veno-arterial extracorporeal membrane oxygenation (ECMO), have permitted more widespread availability of circulatory support for CS.
There is a paucity of data on trends in utilisation and outcomes of MCS use in CS3, incorporating recent technological improvements in therapies. Also, the extent to which changes in MCS use have paralleled overall rates of CS is unknown. Thus, we aimed to identify recent national trends in MCS utilisation in the USA, patient-level predictors of MCS use, and in-hospital outcomes in CS inclusive of ECMO.
Methods
STUDY POPULATION
All hospitalisations of adults (≥18 years old) with a diagnosis of CS (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM], code 785.51) in any position in the National Inpatient Sample (NIS) from January 2004 up to December 2014 were included. The NIS is the largest publicly available all-payer inpatient healthcare database in the USA, representing a 20% sample of all US hospital discharges4. Studies in the NIS are exempt from Institutional Review Board approval at the Beth Israel Deaconess Medical Center.
EXPOSURES AND OUTCOME
The primary exposures of interest (Supplementary Table 1) were short-term non-durable MCS (ND-MCS), intra-aortic balloon pump (IABP), centrally cannulated extracorporeal membrane oxygenation (ECMO), and peripherally cannulated ECMO or percutaneous cardiopulmonary support (PCPS), collectively defined as MCS. ND-MCS would typically include percutaneous devices (TandemHeart™ [CardiacAssist Inc., Pittsburgh, PA, USA] and Impella® devices [Abiomed]), as well as non-percutaneous devices (Thoratec paracorporeal ventricular assist device [Thoratec Inc., Pleasanton, CA, USA], AB5000 [Abiomed], BVS 5000 [Abiomed], and CentriMag® [Thoratec Inc.])3.
Outcomes (Supplementary Table 1) included the proportion of CS hospitalisations using MCS, costs, receipt of coronary angiography, percutaneous coronary intervention (PCI), coronary artery bypass grafting (CABG), left heart catheterisation, and right heart catheterisation, rates of cardiac arrest, major bleeding, cerebrovascular accident, and in-hospital mortality5-7. Direct in-hospital costs were analysed in aggregate and stratified annually using Healthcare Cost and Utilization Project (HCUP) cost-to-charge ratios, adjusted for inflation using the consumer price index inpatient hospital services inflation multiplier with 2014 as the base year. Hospitalisation for CS was the primary unit of analysis with unweighted discharge estimates presented.
COVARIATES
Patient-level covariates included age, sex, hospital bed size, and 18 clinical and procedural variables previously associated with mortality in CS (Supplementary Table 2)3,8-17. We stratified hospitalisations by comorbid discharge diagnoses clinically associated with CS, including non-ST-segment elevation MI (NSTEMI), ST-segment elevation MI (STEMI), myocarditis, pulmonary embolism, hypertrophic cardiomyopathy, and infective endocarditis. We additionally determined the top ten primary discharge diagnoses associated with MCS utilisation in CS by device type. Stratification by states or regions was not examined, due to annual variation in sampling of hospitals within states prior to 2012 in the NIS.
STATISTICAL ANALYSIS
We evaluated rates of MCS use over the study period overall and stratified by type of device and comorbid discharge diagnoses. Patient characteristics by MCS receipt were compared via t-tests and chi-square tests. The Mantel-Haenszel trend test was used to assess temporal trends in MCS utilisation by device type. Total direct hospitalisation costs were determined in 2014 US dollars, stratified by type of therapy, using the HCUP cost-to-charge ratio. A multivariable marginal model, accounting for the complex sampling design in the NIS, was used to determine predictors of in-hospital mortality in MCS recipients using all available variables and a logit link. All analyses were carried out with SAS version 9.4 (SAS Institute Inc., Cary, NC, USA) at a two-tailed p-value <0.05.
Results
OVERALL RESULTS
A total of 183,516 hospitalisations (906,382 weighted to national estimates) with CS were included, of which 47,636 (235,048 [25.9%] weighted) received MCS (Table 1). MCS recipients were younger and less frequently female. More than two thirds (71.6%) of MCS recipients had a diagnosis of MI versus fewer than half (42.9%) of non-recipients (p<0.0001). Among comorbid diagnoses evaluated, rates of MCS use were highest for STEMI (49.6%) followed by myocarditis (41.0%), NSTEMI (26.9%), infective endocarditis (18.7%), hypertrophic cardiomyopathy (17.8%) and pulmonary embolism (12.1%). Most (68.1-73.3%) CS hospitalisations occurred at large hospitals regardless of MCS receipt. The frequency of hospitalisations for CS increased by 214.4% (11,421 to 24,489), disproportionate to MCS use (3,193 to 5,110 [160.0%]). Correspondingly, MCS as a proportion of CS hospitalisation declined starting in 2008 (29.5% to 20.9%).
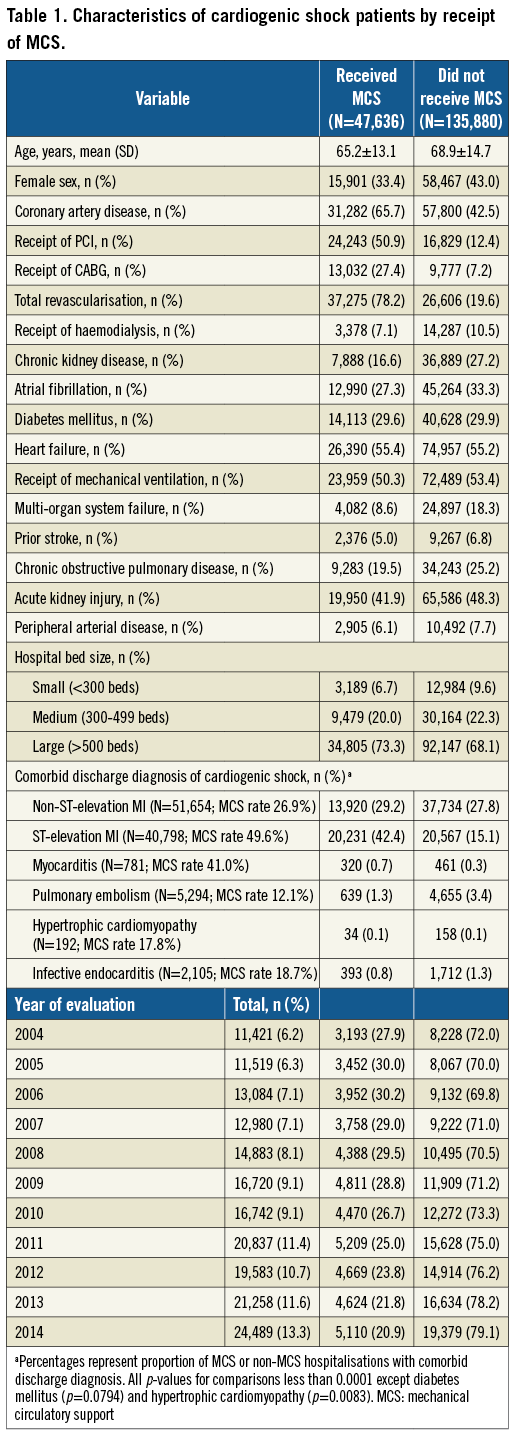
TYPE OF DEVICE
IABP was the most commonly used device (91.3% of MCS), followed by ND-MCS (5.4%), ECMO (3.2%) and PCPS (0.1%) (Table 2). While the overall number of devices increased across all categories (Figure 1), relative IABP utilisation declined as a proportion of overall MCS use (Figure 2), starting in 2008, concomitant with a rise in other MCS use (ND-MCS, IABP, ECMO: p-value for trend <0.0001; PCPS: p-value for trend=0.2035). Growth of MCS was highest for ECMO (1,421.4%), followed by ND-MCS (1,229.2%), PCPS (216.7%), and IABP (140.5%). Within the ND-MCS category, rates of increase were highest for percutaneous devices (5,830.0%) versus non-percutaneous devices (129.8%). IABP was the most commonly used device in MI, utilised in 92.9% of CS with concomitant non-ST-elevation MI (NSTEMI) diagnosis and 94.0% with STEMI. NSTEMI (ICD-9-CM 410.71) represented the most common discharge diagnosis associated with MCS (18.9% of hospitalisations using IABP, 17.3% using ND-MCS, 7.5% using ECMO, and 2.8% using PCPS) (Supplementary Table 3).
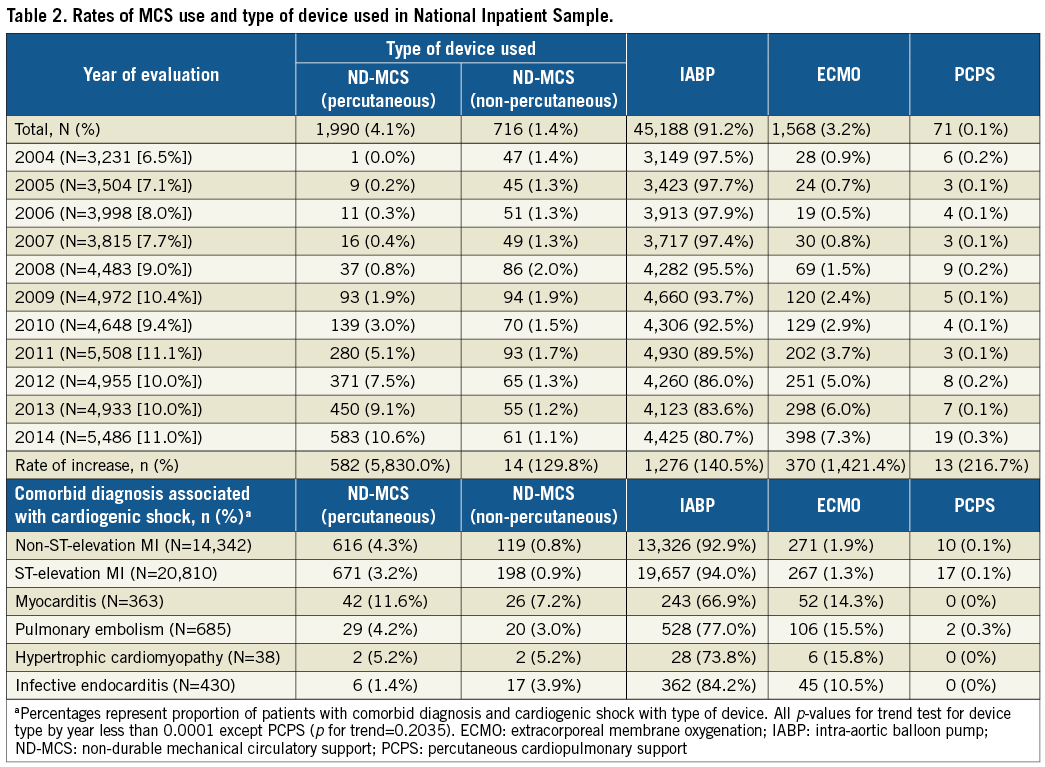
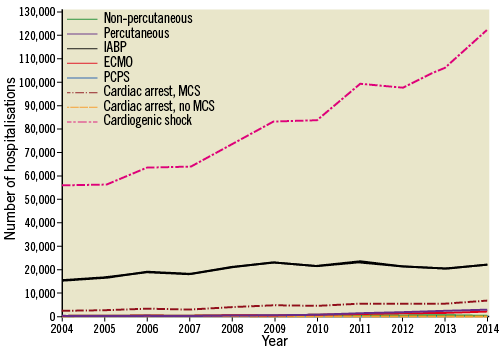
Figure 1. National estimates of the utilisation of mechanical circulatory support devices in individuals with cardiogenic shock, stratified by device type, 2004-2014. Numbers are weighted at the level of hospitalisation to reflect national estimates of utilisation. Cardiac arrest, MCS: rates of cardiac arrest in individuals receiving MCS; Cardiac arrest, no MCS: rates of cardiac arrest in individuals not receiving MCS; ECMO: centrally cannulated extracorporeal membrane oxygenation; IABP: intra-aortic balloon pump; Non-percutaneous: non-percutaneously implanted non-durable mechanical circulatory support device; PCPS: peripherally cannulated extracorporeal membrane oxygenation; Percutaneous: percutaneously implanted non-durable mechanical circulatory support device
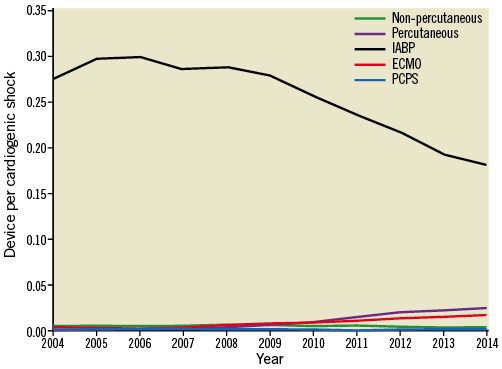
Figure 2. Proportion of cardiogenic shock hospitalisations receiving mechanical circulatory support by device type, 2004-2014. ECMO: centrally cannulated extracorporeal membrane oxygenation; IABP: intra-aortic balloon pump; Non-percutaneous: non-percutaneously implanted non-durable mechanical circulatory support device; Percutaneous: percutaneously implanted non-durable mechanical circulatory support device; PCPS: peripherally cannulated extracorporeal membrane oxygenation
UNADJUSTED OUTCOMES
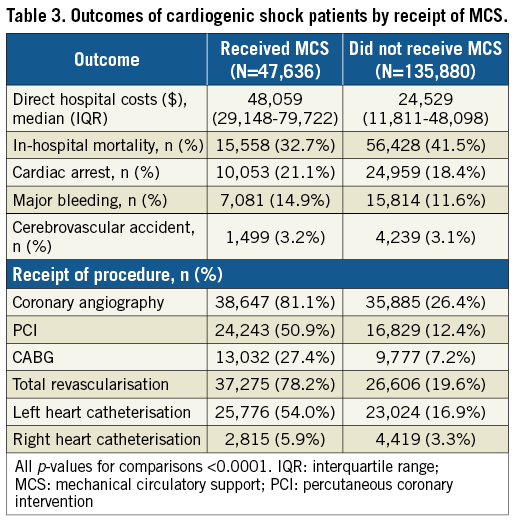
Rates of concomitant cardiac procedures were higher in MCS recipients (Table 3), with half (50.9%) receiving PCI and one quarter (27.4%) receiving CABG. Right heart catheterisation rates were low (MCS vs. non-MCS 5.9% vs. 3.3%; p<0.0001). Cardiac arrest (21.1%) and major bleeding (14.9%) were more common in MCS recipients. There were no significant differences in rates of stroke between MCS (3.2%) and non-MCS recipients (3.1%; p=0.77). There were 15,558 in-hospital deaths in the MCS group versus 56,428 in the non-MCS group (in-hospital mortality 32.7% vs. 41.5%, respectively; p<0.0001). In-hospital mortality declined in both groups from a peak of 37.6% in 2004 for MCS recipients and 52.1% for non-MCS recipients to 33.5% and 38.9% in 2014 for MCS vs. non-MCS recipients, respectively (Supplementary Figure 1).
ADJUSTED OUTCOMES
Multivariable predictors of in-hospital mortality in the subgroup receiving MCS are presented in Supplementary Figure 2. Age was associated with a 35% increase in odds of in-hospital mortality per decade (adjusted odds ratio [AOR] 1.35, 95% CI: 1.32-1.37, p<0.0001). Female gender was associated with a 15% increase in odds for in-hospital mortality (AOR 1.15, 95% CI: 1.10-1.20, p<0.0001). The highest risk conditions were cardiac arrest (AOR 2.05, 95% CI: 1.94-2.16, p<0.0001) and receipt of mechanical ventilation (AOR 1.89, 95% CI: 1.80-2.00, p<0.0001). The adjusted odds of in-hospital mortality for patients receiving MCS (using year 2004 as reference) declined until 2008 and were constant thereafter (Supplementary Figure 3).
COSTS OF HOSPITALISATION
Overall direct hospitalisation costs for MCS patients were $23,530 higher (MCS vs. non-MCS median [IQR] cost in 2014 US dollars: $48,059 [29,148-79,722] vs. $24,529 [11,811-48,098]; p<0.0001) (Table 3). This relationship remained constant over the study period.
Discussion
Despite rising rates of hospitalisation for CS, recent national trends in MCS use have not been evaluated. Our study demonstrates that CS hospitalisations increased faster than MCS utilisation. Additionally, there was a decline in IABP use relative to other forms of MCS starting in 2008. Over two thirds of MCS recipients had a concomitant diagnosis of MI. Procedures were more common in MCS recipients, though rates of right heart catheterisation remained under 6%, regardless of therapy. Hospitalisation costs were greater for MCS recipients and remained stable. In-hospital mortality for CS declined over the study period, with greater declines in MCS recipients, remaining above 30%.
These data suggest that physicians may be using MCS less frequently in CS, or that CS admissions are increasing faster than the current capacity to place MCS devices. Additionally, physicians are increasingly selecting ND-MCS, particularly percutaneous devices, and ECMO in lieu of IABP. The observed relative decline in utilisation may reflect growing uncertainty about the importance of IABP in CS, given the publication of multiple trials from 2007-2012 suggesting the lack of efficacy of IABP in patients presenting with MI complicated by CS18-20. As a result, the 2017 European Society of Cardiology guidelines for management of patients with acute myocardial infarction (AMI) downgraded IABP use to class III for use in CS21. Regardless, it remained the most common MCS device, with usage rates nearly sevenfold higher than the next most common device. While the majority of CS hospitalisations were managed at large hospitals, it remains unclear whether the observed relative decline in utilisation of MCS is due to a hospital’s inability to offer MCS to individuals presenting with CS. This merits further research.
Second, while safety and efficacy have been demonstrated previously for multiple aetiologies22-25, utilisation of MCS for CS outside of MI was less common, with rates of use of 41.0% in myocarditis and 12.1% in pulmonary embolism complicated by shock. The Extracorporeal Life Support Organization (ELSO) multicentre registry has reported survival to hospital discharge as high as 67% in myocarditis patients receiving ECMO, suggesting an expanded role for ECMO in short-term support26,27. Data are more limited to support its use in pulmonary embolism28. The infrequent utilisation of MCS in these settings may suggest an opportunity to improve upon the haemodynamic support of patients with non-MI conditions and shock, though optimal use needs to be further defined.
Third, MCS recipients had lower rates of comorbidities and more frequently received cardiac procedures. While this finding may reflect an appropriate response to the perceived futility of such interventions for the highest acuity patients, it suggests that MCS patients receive more aggressive care overall as part of a “procedural bundle”. Thus, the lower in-hospital mortality seen in MCS patients may not only reflect the underlying selection of healthier patients for more aggressive treatment but also the impact of other interventions. Hospitalisation costs were $23,530 higher in MCS recipients, which may reflect differences in patient and hospital characteristics, the costs of the MCS device, and concomitant interventions. Of note, right heart catheterisation was uncommon with overall rates <6%. This finding may possibly reflect emergent MCS implantation or scepticism about the role of right heart catheterisation29-31. Whether more routine use of right heart catheterisation in this setting would improve overall shock outcomes is unknown and warrants further evaluation.
Fourth, despite improvements in CS care22,32, survival remains very low, with over 30% of patients dying prior to discharge regardless of receipt of MCS. While in-hospital mortality declined in both MCS and non-MCS recipients, survival plateaued after 2008. The proportional decrease in mortality over this time period may be due to secular trends in the management of cardiogenic shock patients. The high and stable mortality in this population, regardless of receipt of MCS, reflects the acuity of this condition and suggests the urgent need for new management strategies.
Limitations
There are several limitations of the current study. First, the study is retrospective and included a limited number of variables, which are probably related, and causality cannot be inferred. Second, the timing of MCS relative to other diagnoses noted on discharge cannot be discerned. Third, inaccuracies in coding may reduce precision and interpretation of the results. Fourth, as the hospitals sampled in the NIS differ annually, no hospital level comparisons could be made across years and may not fully reflect national trends. Lastly, as costs were derived from hospital charges, they may not represent the total direct or induced costs or cost-effectiveness of MCS therapy.
Conclusions
Despite improvements in survival for CS over time, in-hospital mortality remains high. Relative utilisation of IABP has declined since 2008, though continues to increase in volume. Hospitalisations for CS have outpaced increases in overall MCS use. MCS use remains uncommon outside of MI presentations and is associated with an overall increase in the rates of concomitant procedures and costs during hospitalisation for CS.
| Impact on daily practice Rates of cardiogenic shock have outpaced MCS utilisation. MCS use remains uncommon outside myocardial infarction with high mortality (32.7%) and high utilisation of other procedures. |
Funding
This work was supported by an investigator-initiated grant from Abiomed, Danvers, MA, USA. Members of the study team are additionally supported by funding from the National Heart, Lung, and Blood Institute (1F32HL1407-11 to J.B. Strom, R01HS024520-01 to C. Shen and 1R01HL136708-01 to R.W. Yeh). The funding organisations had no role in the final edits or submission of this manuscript.
Conflict of interest statement
J. Popma reports grants from Medtronic, Abbott Vascular, and Direct Flow Medical and personal fees from Boston Scientific, Cordis, and Direct Flow Medical, outside the submitted work. D. Pinto reports personal fees from Medtronic, The Medicines Company, Medicure, and Abiomed, outside the submitted work. R. Yeh reports investigator-initiated grant funding from Abiomed related to the conduct of the study, grant support from Boston Scientific, and consulting fees from Abbott, Medtronic, and Teleflex, outside the submitted work. The other authors have no conflicts of interest to declare.
Supplementary data
Supplementary Table 1. Administrative codes used to define exposures, major covariates, and outcomes.
Supplementary Table 2. Comorbid conditions adjusted for in the regression model and representative administrative codes.
Supplementary Table 3. Top ten primary discharge diagnoses in hospitalisations involving mechanical circulatory support and cardiogenic shock by device type.
Supplementary Figure 1. Proportion of hospitalisations resulting in death by receipt of mechanical circulatory support, 2004-2014.
Supplementary Figure 2. Multivariable predictors of in-hospital mortality in the subgroup receiving mechanical circulatory support.
Supplementary Figure 3. Adjusted odds ratio for likelihood of in-hospital mortality by year in patients receiving mechanical circulatory support.
To read the full content of this article, please download the PDF.

